Introduction :
Ammonia needs to be monitored in drinking water to safeguard human health. It can be present in water fish water also, there is need to check ammonia level in fish water for maintain level of it. We bought Ammonia analytical kit last year. firstly is need to calibrate the machines to the primary standard for check quantitative accurate values for it.
Objective :
To calibrate ammonia ISE (Ion selective electrode)for quantitative analysis of ammonia in fish water, waste water.

WHAT IS AMMONIA ISE ION SELECTIVE ELECTRODE ?
Ammonia gas selective electrode is a combination electrode designed for the measurement of ammonia in aqueous solutions such as waste water samples.
| Type | NH3 gas sensing electrode with glass pH internal, Ag/AgCl reference and gas permeable PTFE membrane. |
| species measured | NH4+ , NH3 |
| measurement range | minimum 0.02 ppm |
| operating temperature | 0 to 40 degree celcius |
| operating pH | >11 pH |
| connection | BNC |

Solutions Required for Calibration:
- lonic Strength Adjuster (ISA): HI 4001-00
- 0.1 M standard NH3
- 100 ppm Ammonia standard
- 1000 ppm Ammonia Standard

Inner electrode check :
Prepare pH test solutions HI 4000-47-4 and HI 4000-47- 7 by mixing and dissolving each buffer packet in separate containers with 50 ml. deionized water. These pH solutions contain chloride ions and pH buffers that are used to verify the inner electrode (pH internal) is operational.

- If sensor has been stored or shipped dry, it should be “conditioned” by soaking the pH/reference electrode 1 hour or more in one of the pH test solutions.
- Avoid touching the pH glass with your fingers.
HOW TO TEST pH ?
- Connect the BNC connector on the electrode cable to a pH/mV (mV or ORP mode) meter.
- Carefully immerse the sensor assembly into one of the buffers.
- When the measurement stabilizes record the mV generated.
- Rinse sensor tip in deionized water and dab dry between buffers to prevent solution carry-over. Do not rub the glass.
- Take a measurement in the second buffer and record mV.
- Pay attention to minus sign if present.
The difference in mV between the two pH solutions.
A calculated value equal or greater than 160 mV is acceptable for ambient temperatures between 20° and 25°C.
HI 4000-47-7 -134.2 mV
HI4000-47-4 62.2 mV
Difference 62.2 – (- 134.2 ) = 196.4 mV > 160 mv

Electrode Preparation :
- Removed glass internal part from sensor body and electrode check as mentioned in above step.
- Installed PTFE ( white membrane like teflon ) on outer prob body with the help of twizzers.
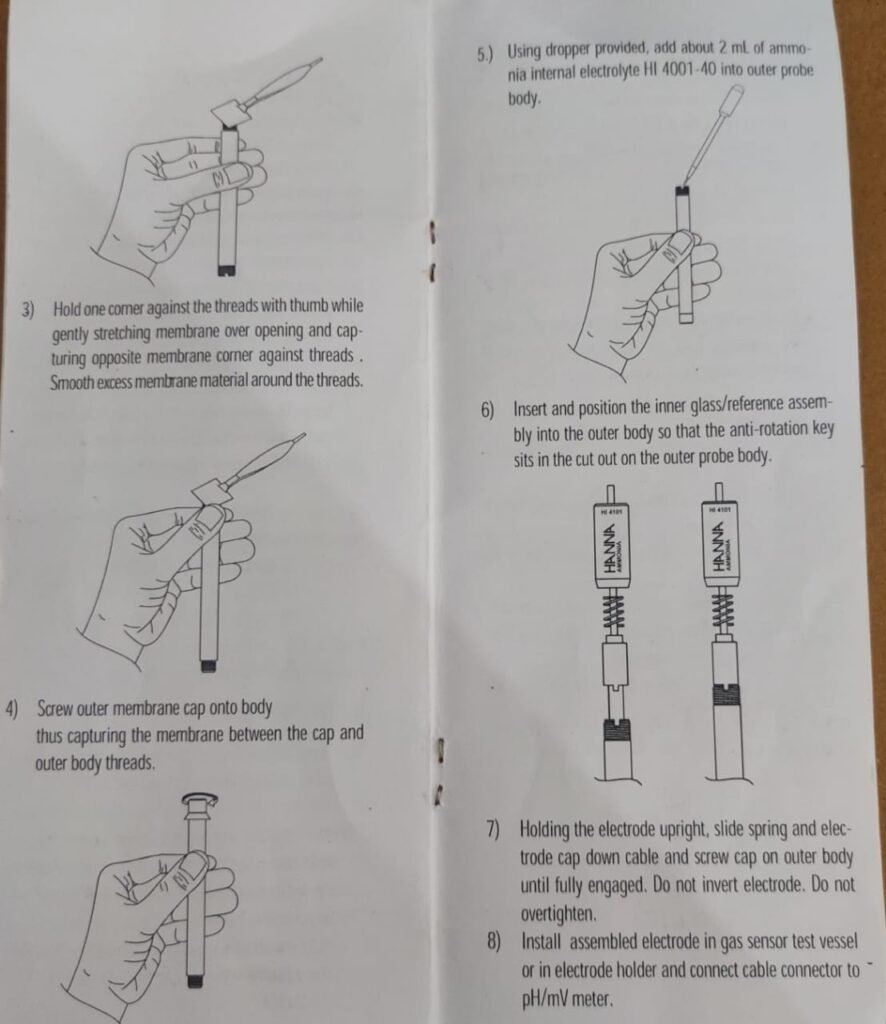
- Holded one corner against the threads with thumb while gently stretching membrane over opening and cap- turing opposite membrane comer against threads.. Smooth excess membrane material around the threads.
- Screwed outer membrane cap onto body thus capturing the membrane between the cap and outer body threads.
- Using dropper provided, add about 2 ml of ammonia internal electrolyte HI 40031-40 into outer probe body.
- Inserted and position the inner glass/reference assem bly into the outer body so that the anti-rotation key sits in the cut out on the outer probe body.
- Holded the electrode upright, slide spring and electrode cap down cable and screw cap on outer body until fully engaged. Do not invert electrode. Do not overtighten
- Installed assembled electrode in gas sensor test vessel or in electrode holder and connectd cable connector to pH/mV meter.
Quick Check of Electrode Slope

- Connected BNC (connector) to pH/mV/ISE meter.
- Placed meter in mV mode.
- Placed 100 ml of deionized water into a vessel with stir bar. Add 2 ml of ISA Hanna HI 4001-00.
- Placed sensor into prepared sample. Add 1 ml of 1000 ppm or 0.1 M Ammonia standard to beaker.
- Recorded the mV value when stable. -74.1 mV
- Added an additional 10 mL of standard to the solution. Recorded the mV -130.1 mV when reading has stabilized. This value should be less than the previous noted (more negative).
- Determined the difference between the two mV values. -74.1 – (-130.1 ) = 56 mV
- acceptable value for this slope is 54±4 mV at ambient temperatures between 20 and 25°C.

Precautions :
- Verify that the upper cap has been screwed in all the way.
- Verify electrode is connected properly to meter and the meter is is powered.
- Verify ISA has been added in the correct ratio to the standard.
- Examine the white membrane and check for electrolyte that might have leaked through the PTFE film. Replace membrane if damaged.
STANDARD PROCEDURE TO EXAMINE SAMPLE
- The volume of the unknown sample (V sample) is measured accurately and placed into the closed sample vessel. The sensor is secured in the vessel and then the vessel is placed on a stirrer.
- ISA is added at 1 part per 50 parts sample.
- When the measurement is stable the mV value is noted.
- A known amount, volume (V standard) and concentration Standard (C standard) of NH, standard is then added to the sample. mV values are again noted when the measurement is stable.
- The mV change is then calculated (ΔE )
- Using the measured and calculated values, the sample concentration (C sample) can be determined.
formula for ammonia concentration

Quick check

Sample checking
- Took 50 ml sample and added 1 ml ISA Ionic strength adjuster.
- recorded the mV
- added 5 ml standard 0.1 M solution
- recorded mV
- find out concentration of sample using formula.

Using Same method We can use Nitrate electrode to check nitrate and nitrite
short method to slope check Place reference and measuring half-cell or combina- tion electrode into prepared sample.Place reference and measuring half-cell or combina- tion electrode into prepared sample.Place reference and measuring half-cell or combina- tion electrode into prepared sample.Place reference and measuring half-cell or combina- tion electrode into prepared sample.Place reference and measuring half-cell or combina- tion electrode into prepared sample.
Checked Azolla water NO3 is about 4.27 ppm And Ammonia is 1.291ppm
HI 4113
The Hanna HI 4113 is shipped disassembled. The sensor is shipped with two HI 4113-51 modules.
1. Unwrap ParafilmⓇ seal found over ceramic junction on inner stem and discard. This is only used for ship- ping or long term storage.
2. Remove sensing cone from shipping vial. Do not touch the sensing membrane with the “H” hole pattern on it.
3. Screw the cone into the inner stem finger tight. Do not over tighten.
4. Rinse inner stem with deionized water making certain to wet o-ring found on the inner stem.
5. Reassemble electrode by gently pushing the inner assembly into the outer body, sliding spring down cable, and screwing cap into place.
6. Remove fill hole cover and o-ring on fill hole spout. Using the dropper pipette provided, add a few drops HI 7078 fill solution to the electrode. Invert the elec- trode to wet the o-ring and rinse the fill solution cham- ber.
7. Holding the body of the electrode gently press upper cap with your thumb. This permits the fill solution to drain out of the body. Release cap and verify elec- trode returns to its original position. (You may need to gently assist for this to occur).
8. Tighten the electrode cap onto the body and fill elec- trode body until fill solution volume is just below fill hole.
9. Position electrode in a Hanna HI 76404 electrode holder (or equivalent) and connect BNC connector to meter.
Quick Check of Electrode Slope
- Connect electrode(s) to pH/mV/ISE meter
- Place meter in mV mode.
- Place 100 mL of deionized water into a beaker with stir bar.
- Place reference and measuring half-cell or combina- tion electrode into prepared sample.
- Add 1 mL of a standard to beaker. Record the mV value when stable.
- Add an additional 10 ml of standard to the solution. Record the mV when reading has stabilized. This value should be less than the previous value noted (more negative).
- Determine the difference between the two mV values. An acceptable value for this slope is 56±4 mV (20-25°C)


Calculation done for Azolla Water using Above formula same as Ammonia Electrode.





