Introduction :
In Vigyan Ashram, We are working on hydroponics and agriculture techniques but one of our hydroponic unit (NFT nutrient film technique) got difficient of magnesium. We provided MgSO4 magnesium sulphate but results are not good. because of bonding of MgSo4 it react with other nutrient or cant be absorb. chelated nutrient are absorbed easily and are quite expensive So we decided to take Survey of Farmers , which fertilizers they use and which are most effective ?
Survey of Farmers :
Talking to the farmers, it was found that farmers use three types of fertilizers which include grade one and chelated micronutrients. Grade one fertilizers are generally applied by drip. About twenty five percent of the expenditure is spent on chelated fertilizers. Chelated fertilizers are around Rs 900 per kg. The highest amount of boron is found in chelated fertilizers. The local brand used by farmers is Purva Chemicals. Chelated fertilizer shows results in 2 days but normal fertilizers are applied for ten days.
Chelated fertilizers are widely applied by spray to tomato farmers costing Rs 2,000 per acre per day of calcium boron. Onion farmers use chelated micronutrient in large scale for plant growth and development. Chelated fertilizers are widely used by farmers But the cost of these fertilizers is huge The companies making chelated fertilizers charge all kinds of expenses, taxes, GST, transportation etc. the price of fertilizer increases very much. Common farmers cannot afford this price, but it is necessary to use chelated fertilizers. therefore we decided to make chelated micronutrient in lab.
SAT , 5 august 2023.


WHAT IS CHELATED MICRONUTRIENT ?
Chelated micronutrients are commonly used in agriculture to improve plant growth and crop yields. By providing essential minerals in a more bioavailable form, chelated micronutrients can help plants grow stronger, healthier, and more resistant to pests and diseases. Chelated minerals, such as chelated iron, zinc, and copper, are used as additives in fertilizers. They enhance the availability of essential nutrients for plants by preventing insoluble metal compounds from forming.
Chelation is a chemical process in which a chelating agent forms coordinated bonds with a metal ion to create a stable complex.
Chelating agents are typically organic molecules with multiple binding sites, known as ligands. These ligands have functional groups, such as oxygen, nitrogen, or sulfur, which can donate electron pairs to the metal ion through coordination bonds. The resulting chelate complex is often more stable than the individual metal ions or ligands.
Thera are two types of chelating agents ;
EDTA : EDTA is a weak acid. Four carboxyl and two amine groups make up EDTA (ethylenediamine tetra acetic acid), This white, water-soluble solid.
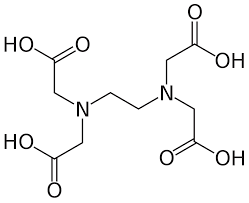
AMINO ACIDS : Amino acids are commonly used in chelation processes due to their ability to form stable complexes with metal ions. They contain functional groups, such as amino (-NH2) and carboxyl (-COOH) groups, Glycine , histadine , cysteine these are several amino acids.
SAT , 12 August 2023
Boron is very common element in fertilizer used by farmers so decided to make chelated boron in laboratory.
Chelated boron is a form of boron that is bound to a chelating agent, which is a molecule that forms a complex with the boron ion, making it more stable and soluble. Chelated boron is often used in agriculture as a micronutrient fertilizer to prevent boron deficiency in plants. Chelated boron compounds include boron chelates with amino acids, EDTA (ethylene diamine tetraacetic acid), and other organic ligands.
Which amino acid used to chelate boron ?
Glycine is often used as a chelating agent to improve the solubility and availability of boron in various applications, particularly in agriculture. Boron deficiency can negatively affect plant growth and development, so chelated boron compounds, including those with glycine, are used as a means of supplying this essential micronutrient to plants.
It’s worth noting that while glycine is a common amino acid used for boron chelation, other chelating agents like ethylenediaminetetraacetic acid (EDTA) and other amino acids may also be employed for similar purposes. The choice of chelating agent depends on factors such as the specific application, the target pH range, and the desired stability and bioavailability of the chelated boron compound.
EDTA IS THE BEST OPTION TO CHELATE BORON
| Material | costing (Rs per kg ) |
| Glycine | 300 -400 |
| EDTA | 50-60 |
| Boric acid | 100-150 |
| DOT disodium octaborate tetrahydrate | 160-220 |
| Sodium borate or borax | 80-100 |
18 August , 2023
| name | village | crop | farm | expenses | cost of chelated fertilizer |
| Santosh Barve | Chas | Soybean , Ginger | 1 acre | 10000/month | 3000/month |
| Dattatray Chaskar | Chas | tomato ,brinjal | 2 acre | 40000 till crop yielded | 8000 |
| Pappu Bhor | Chas | Tomato | 1 acre | 30-40000till crop yielded | 10000 |
| Sachin Chaskar | Kadewadi | soybean | 1 acre | 15000/month | 2000/month |
| Vikas Vare | Gulani | onion | 1 acre | 5000/week | 1000/week |
| Ramkrushn Dherange | Gulani | Sugarcane | 2 acre | 40000/month | – |
| Vishal Wayal | Gulani | onion , tomato | 2 acre | 20000/month | 3000/month |
| Godekar | Pabal | tomato | 1 acre | 25000/month | 2000/month |
| Rahul Chaudhari | Pabal | sugarcane | 1 acre | 25000/month | – |
| Ravindra Gulve | Gulani | tomato | 1/2 acre | 20000/month | 1800/month |
| Vishal Gulve | Gulani | onion | 1 acre | 8000/month | – |
| Bhausaheb Chaudhari | Pabal | Bajara | 1/2 acre | 1500 /month | – |
| Arjun Chaudhari | Pabal | Moong | 1/2 acre | 1500/month | – |
| Santosh Kadam | Perne Phata | Sugarcane | 3 acre | 40000/month | 10000/month |
| Sachin Bhor | Chas | Ginger | 1 acre | 8000/month | 1000/month |
| Vaibhav Vavhal | Pargoan Shingave | tomato , Bitter guard | 1 acre | 40000 till crop yeilded | 15000 |
I talked with 16 farmers in various areas near Pabal village. It conclude that Farmers mostly use Mahadhan 24:24:00 , 13:00:45 10:26:26 like various fertilizers. Most common Chelated fertilizer include all elements like Copper , zinc , boron , Molybdenum , Manganese , Ferrous etc. not applied separately element only tomato farmers use calcium boron separately for plant fruit growth purpose , mostly farmers used drip irrigation instead of Foliar spray because labors charges include in foliar spraying.


these are some Chelated micronutrient fertilizer brands

19 August , 2023
On 19 August , visited Krishi Vigyan Kendra , Narayangaon’s Soil testing labs discussed with Mr. Yadav sir about Fertilizer and its application , need of chelated fertilizer for better crop yield ,

As per Discussion with Yadav sir , fertigation should be integrated. there are 3 types in fertigation technique includes Organic fertilizers , chemical fertilizers , biological fertilizers.
| fertilizer Type | % use | examples |
| Organic | 30 | compost ,vermiculture , bonemeal , karanjpendh , greens fertilizer |
| synthetic /chemical | 50 | urea , Single super phosphate |
| Biological | 20 | bacteria culture , phosphate solubilizing bacteria , potash mobilizing bacteria |
25 August , 2023
Objective :
- Synthesis of Magnesium Chelate for better plant absorption.
- Find Alternative methods from Agriculture based Chelation like amino acids / Bacterial culture
- Quantification of chelated and non chelated as well as precipitated Magnesium in Hydroponic systems.
NEED FOR synthesis of magnesium chelate

We are working on hydroponics and agriculture techniques but one of our hydroponic unit (NFT nutrient film technique) got difficient of magnesium. We provided MgSO4 magnesium sulphate but results are not good. so We decided to make Magnesium chelate in laboratory.
Quantification of Magnesium :
Procedure for Determination of Magnesium :
Chemicals Required :
- 0.1 M EDTA disodium Solution : Take 7.44 gm of EDTA in 200 ml Distilled water.
- Eriochrome Black T indicator : 0.2 gm of EBT with 20 ml ethanol.
- Buffer Solution : 57 ml Ammonia NH3 + 7 gm of Ammonium chloride NH4CL diluted in 100 ml distilled water.
- Conical flask 250 ml
- Burette
- funnel
- measuring cylinder
- weighing balance
Procedure :
- Take 10 ml sample including magnesium in conical flask.
- add 5 ml buffer solution.
- add 3-4 drops of EBT indicator.
- Fill the burette with 0.1 M EDTA solution and titrate solution of magnesium with EDTA.
- Record the reading and calculate concentration of Magnesium.
27/08/2023
Trial 1 for Magnesium :
- Took 1 liter each in beaker BARC ( Kitchen waste Culture) , Agri. compost Culture , Normal water.
- Added 5 gm of Magnesium Sulfate in each Beaker.
- titrate these solution against 0.1 M EDTA solution and recorded reading.
- kept for 24 Hours.
- After 24 Hours Titrate these solution against 0.1 M EDTA solution.and recorded reading.
5 gm Magnesium sulfate contains 9.8 % of Magnesium Mg.
MgSO4.7H2O Magnesium sulfate heptahydrate 246.48
Molecular weight
Mg = 24.3
S (sulfur) = 32.06
O ( oxygen) = 16
H ( Hydrogen ) = 1
24.3+32.06+(16 x 4 ) + 14+ 112 = 246.48
in 246.48 of Magnesium sulfate contains 24.3 grams of Magnesium
100 gm contains 9.8 gm of Magnesium
| Sample | Titration reading on 27/08/2023 | After 24 Hours |
| Normal Tap Water | 2.3 ml for 10 ml sample | 2.2 ml |
| Agri. compost Cultutre | 2.7 ml | 3.3 ml |
| BARC | 2.6 ml | 3.2 ml |
0.1 M EDTA solution ( 200 ml )contains 7.44 gm of EDTA
200 ml = 7.44 gm
2.3 ml = ? 0.085 gm of EDTA
372.24 gm of EDTA is Equivalent to 24.3 gm of Magnesium
0.085 gm of EDTA contains = 0.085 x 24.3 / 372.24 = 5 mg of magnesium in 10 ml of sample (Normal water)
and 1000 ml sample solution contains 500 mg of Magnesium
We added 5 gm of Magnesium sulfate in 1000 ml of sample solution which contains 9.8 % means 490 mg of Magnesium.
Content of Magnesium calculated by using above method for following.
| sample | content of Magnesium | content of Magnesium after 24 hours |
| Tap Water | 500 mg | 500 mg |
| Agri. compost culture | 600 mg | 700 mg |
| BARC culture | 600 mg | 700 mg |
Conclusion : We added 5 gm of Magnesium sulfate in 1000 ml of sample solution which contains 9.8 % means 490 mg of Magnesium. If chelate of Magnesium was formed in culture then content of magnesium get decreased because of chelation process. but when we determined content of magnesium after 24 hours it increases. its because of contaminants in MgSO4 powder ( Calcium , Ferrous etc.) so we decided to take another trial using calcium solution using Magnesium indicator to check contaminants are the reason of higher magnesium content in samples of cultures.
TRIAL 2 for calcium using EBT Eriochrome black T indicator ( magnesium )
Took 200 ml Distilled Water and added ( 2 gm of Calcium carbonate and 1 ml Hydrochloric acid ) the titrated the solution against 0.1 M EDTA Solution.
this trail shows endpoint means calcium is detected using magnesium indicator . TRIAL 2 for calcium solution using EBT Eriochrome black T indicator ( magnesium ) shows same results like magnesium determination .here conclude that chelate was not formed using agri. compost culture and BARC culture
05 September , 2023
Trial 3
Trial 3
To check magnesium sulfate is precipitated in Hydroponic water or not ?
Solubility of Magnesium Sulfate = 113 gm / 100 ml
Solubility of 12:61:00 Monoammonium phosphate = 32.8 gm / 100 ml
Various Fertilizers used in Hydroponic like Calcium nitrate , Monoammonium sulfate , Magnesium sulfate etc. to grow spinach. if magnesium react with other fertilizers then it gets precipitated and becomes insoluble in water. because of Insolubility plants didnt uptake that Magnesium from Water.
Procedure to check magnesium sulfate is precipitated in Hydroponic water or not ?
- Took 100 ml water in a beaker. Added 10 gm of Monoammonium sulfate 12:61:00 by calculating solubility.
- Added 10 gm Magnesium Sulfate in same Water
- Observe it precipitated or not ?
- After 2 Hours Evaporated the Solution using hotplate to check Salt content
- weighed salt content.
Salt residue = 15 gm
We added 20 gm but MgSO4 have 7 water molecules in it 5 gm get evaporated .
Magnesium was not precipitated, both fertilizers was soluble in water. that means Magnesium is less in Hydronic Water is the reason of Magnesium deficiency. For magnesium deficiency we can use Magnesium Sulfate as a cheap source instead of Chelated Magnesium. so we decided to make Chelated Boron.
8 September , 2023
BORON EDTA Preparation
Chelated boron compounds include boron chelates with amino acids, EDTA (ethylene diamine tetra acetic acid), and other organic ligands.
Materials and Equipment:
- Boron source (such as boric acid or borax)
- Chelating agent (such as EDTA or amino acids)
- Water
- Stirring equipment
- pH meter,
- HCL hydrochloric acid
- Heating equipment
Steps:
- Preparation of Chelating Solution EDTA
- Preparation of Boron Solution: Dissolve the boron source (borax) in water.
- Combining Solutions: Slowly add the chelating solution to the boron solution while stirring continuously. The pH of the solution may need to be adjusted 8 to 10.
- Chelation: Continue stirring the mixture while adjusting the pH as needed. Chelation occurs as the chelating agent binds with the boron ions, forming stable chelated boron complexes.
- Filtration: Once the chelation process is complete, you might need to filter the solution to remove any undissolved particles or impurities.
- Drying.
Boron EDTA Trial 1 17/09/2023
Solubility of Sodium Borate = 31 gm / liter
Solubility of EDTA = 96 gm/liter
pH adjustment 8 to 10
Temperature 25 degree Celsius
Sodium borate decahydrate is a mineral, and a salt of boric acid. The term borax is used for a number of closely related minerals or chemical compounds that differ in their crystal water content, but usually refers to the decahydrate. Sodium borate decahydrate is a mineral, and a salt of boric acid.
Procedure :
- Took a beaker and added 100 ml of Distilled Water. then added gm of EDTA. mixed well.
- Took another beaker and added 100 ml of distilled water then added 3 gm of sodium borate and mixed well
- mix this two solutions slowly by stirring.
- adjusted the pH using hydrochloric acid.
Trial 2 = 20 September 2023
Preparation of Solution :
SOLUTION 1 : 100 ml distilled water + 3 gm Sodium Borate 2. 100 ml Distilled Water + 3 gm EDTA mixture of this two solutions
SOLUTION 2 : 100 ml distilled Water + 3 gm EDTA
SOLUTION 3 : 1 gm of Calcium carbonate + 100 ml distilled Water + 1 ml HCL
Eriochrome Black T indicator
Solution 1 and solution 2 Titrate Against Calcium carbonate Solution. and Recorded the burette reading.
| Sample | burette reading |
| 100 ml distilled water + 3 gm Sodium Borate mix with 100 ml Distilled Water + 3 gm EDTA mixture of this two solutions | 32.8 ml |
| 100 ml distilled Water + 3 gm EDTA | 7.9 ml |
32.8 – 7.9 = 24.9 ml
this trial showed that Chelation of Boron takes placed. 25 % of unreacted EDTA is react with Calcium during Titration. that’s why it shows increase in burette reading. After Chelation ligand is Formed and it didn’t react with other materials.
Trial 3 22/09/2023
Repeated trial 2 but sodium borate is 4 gm for confirmation of chelation of Boron Takes place or Not ?
48.2 ml of Calcium Carbonate solution required for Titration that means 40 % of unreacted EDTA react with Calcium.
Chelation Of Boron Using Low Cost Alternatives

Took chelation trial using gliricidia , subabul , moh
boil 1 liter water with 200 gm of gliricidia
Drain the material and use the water for chelation of boron.
Conclusion:
Best results got in gliricidia than subabul and moh .
gliricidia is best low cost alternative for chelation of micro nutrient.
BORON DEFICIENCY:

planted 10 cauliflower saplings on 16/10/2023 for testing of low cost chelated fertilizer using gliricidia.
When deficiency observed then we will test fertilizer on it.
After one month deficiency not seen on any plant so decided take trial in only water
On 4 December 2023

After 6 days only in water

after 12 Days there was many deficiencies found on plants and plants gets weak.
Parallelly Planted 10 plants in soil and 4 in rocks and stones for getting boron deficient plant.

Making of Boron chelated fertilizer using gliricidia :
- Boil 200 gm gliricia with 1000 ml of water in pressure cooker.
- then drain out water containing amino acid
- Added 20 gm of sodium borate
- cool it and use to spray on plants.

Spraying of Fertilizer


Spraying for 20 Days and plants get deficient


Conclusion :
Boron deficient plant was not found. plants found with various deficiencies. spraying of the fertilizer does not gives result effective here We conclude that we failed to obtain boron deficiency but got Low cost chelating agent as a gliricidia.





