15/2/21
Need of the project:-
Toilet wastewater is the main source of nutrients in household wastewater, and the major fraction of nutrients is found in urine . At least 80% of the nitrogen, 55% of the phosphorus, and 60% of potassium in domestic wastewater originate from urine, and it only occupies less than 1% of the total wastewater volume. In order to maximize nutrient recovery from wastewater, urine should be separately collected and processed.
The mixture of urine and flush water is transported from toilet to the collection tank by separate sewage pipes. When the collection tank is full, urine is transported to a storage tank . During the transportation and storage, urea is hydrolyzed to ammonium and bicarbonate ions, and dissolved ammonia concentration increases. Urine separation provides a range of benefits such as nutrient recycling, decreased water consumption and increased energy efficiency. However, current urine separation systems are faced with problems such as the unstable behavior of ammonia and the large amount of urine which needs to be transported and stored. One strategy to eliminate the problems mentioned above, is to recover the nutrients by processing the urine.
In the past decade, the focus of wastewater engineering has shifted from protecting human health and pollution control to sustainability and resource recovery. Resources such as ammonia and phosphate are essential for human food supply. Ammonia is mainly produced from the Haber-Bosch process, conducted under high pressure and temperature, which consumes more than 1% of the world’s total energy. Phosphorus is abundant in the earth’s crust but its accessibility and quality are limited; Morocco and China alone account for 78% of the world phosphate rock reserves . Price of phosphate rocks had increased 2 to 3 folds in the last decade and the production of high-quality phosphorus reserves is estimated to peak in 20 to 70 years , where phosphorus supply starts diminishing.
After consumption of crops by humans, almost 100% of the nitrogen and phosphorus consumed are released within the excreta and discharged into our sewage systems. To avoid eutrophication within natural water bodies, wastewater treatment plants (WWTPs) are designed to remove these nutrients. The most common approach for removing nutrients from wastewater is by conventional nitrification/denitrification and metal salt precipitation, by which the nutrients are effectively removed but also lost. Nitrification is also an energy-intensive process that consumes up to 60% of the operational cost . A more sustainable approach is a closed nutrient loop that recycles the valuable resources from human waste rather than treating them as an unwanted residual.
Human urine has tremendous potential for nutrient recovery as it contributes to most of the nutrient loads (80% of TN and 45% of TP) but yet accounts to less than 1% of the volumetric flow to a WWTP.
A coherent approach would be to collect the urine separately from the feces then recover the nutrients from the urine, which circumvents dilution and the need for solids/liquid separation in downstream processing. Separated urine collection allows resource recovery from a more concentrated nutrient stream, offers more flexible recovery strategies and alleviates nutrient loads on WWTPs
Objectives:-
- To Develop a system for storage of Urine at Vigyan Ashram, Pabal.(Atleast 3 Months)
- To Recover Nutrients Like Nitrogen, phosphorous, potassium and Magnesium
- To reuse urine water for back flushing with little or no dilution.
- To develop a system where halophilic plants or Plants like palak, lettuce, tomato could be grown using the nutrients from the Urine itself.
- To Achieve economic feasibility for the storage and treatment of urine water at Vigyan ashram, Pabal.
Problems and possible Solutions:-
1) Urine contains a lot of Nitrogen in the form of urea and salts in the form of Sodium chloride. Treatment of urea to generate nitrates biologically looks a viable solution and some amount of nitrogen which would go for cell building rather metabolism of the nitrifying bacteria. Treatment of salts would be done by growing plants who are more halophilic i.e can grow in high salt containing regions.
2) Proper and essential bacterial growth. To Achieve efficient nitrification there should be some amount of nitrifying organisms that we can grow by giving additional amounts of carbon and other nutrients like calcium, molybdenum, sulphur etc required for its cell building.
3) Finding the essential carbon source. Urine has a C:N ratio of 1:1. At this level growth of organisms is very difficult and therefore we need to give some additional amount of carbon. So we need to decide if the carbon source should be easily degradable, mediumly degradable or difficult degradable i.e the carbon source should be glucose, wheat straws or lignin respectively.
4) After treatment of urine water it could be used for flushing. Firstly this urine if required would be diluted with water and further it would be used for flushing. Later there would be no need for the urine dilution, The urine would be directly used for flushing after the treatment process.
5) A lot of ammonia could be lost via volatilization. To stop this from happening acidification with nitric acid, Sulphuric acid, phosphoric acid with proper dosage to keep the ph at such a level where the urease enzyme responsible for decompostion of urea could be inhibited, will be adopted.
20/2/21
Methods for Urine treatment:-

These are list of methods for urine treatment. the numbers mentioned range from 1 to 5, with 1 being the least possible solution to the problem, and 5 being the most viable solution to the problem. Keeping all points into consideration, 3 methods were finalised. Hydroponics, Composting, Ammonia Stripping.
22/2/21
Storage of Urine:-

A 50 Litre Storage drum for collection of urine was setup Inside The Urinal at vigyan Ashram, pabal. Nitric Acid (65%) was dosed at the beginning of the experiment, so the ammonia dosen’t volatize out of the system. the pH was tried to be maintained at 1-3.
25/2/21
Nitrogen Removal:-
Nitrification:-
Experimental Setup:-
2 experiments with a capacity of 2 lit were setupat soil lab vigyan ashram, pabal. the first experiment had a dilution factor of 1:4 (500ml urine and 1.5 lit water). Aeration was given via air pumps with specificatons( 2W, 2L/Min). MBBR were added to the system.To it BARC culture was added after 3 days of experiment. 2nd experiment was of Concentrated urine with no diltion. Aeration was given via the air pump with similar specifications, MBBR were added to the system and BARC culture was added on the first day itself.
5/3/21
Results:-
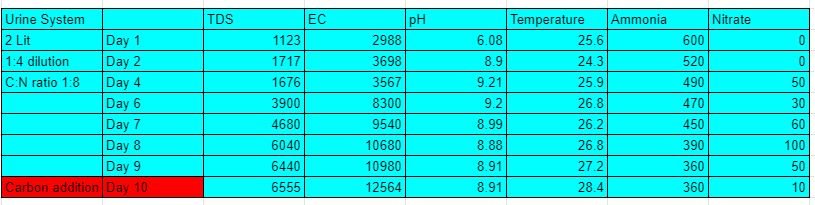
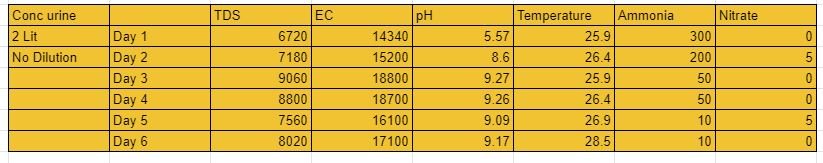
3/3/21
Ammonia Stripping:-
Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it.
Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction.
Stripping is a physicochemical process wherein a liquid mixture is contacted with a gas to remove a volatile component by mass transfer from the liquid to the gas phase . The reverse of the process is defined as absorption. When water is used as an absorber, the gas is dissociated because of its solubility, and the process is commonly referred to as physical absorption. When absorption is accompanied by chemical reaction in the liquid phase, the process is referred to as chemical absorption or reactive absorption. Absorption with chemical reaction is advantageous, because chemical reaction increases absorption capacity of the liquid and the rate of absorption. Stripping and absorption processes are
based on the equilibrium between the gas and liquid phase. In both of the processes, solubility of the solute determines the liquid-to-gas ratio. In the absorption process, preference is given to liquids with high solubilities
for the solute, and in the stripping process, a low solubility of the solute is desired. For a low volatility compound such as ammonia, stripping
and absorption processes are effective only if the stripping conditions are well optimized.
Ammonia is very soluble in water (34% (w/w) at 20°C). Therefore, sufficiently high air flow rates should be used in air stripping. In the case of absorption, solubility of ammonia is an advantage only if the pH is sufficiently low . Although ammonia stripping and absorption are wellknown processes, environmental application of these processes
is rare.
Design criteria:-
The following criteria should be considered when designing ammonia stripping systems.
- Hydraulic wastewater loading
- Stripping air flow rate
- Packing depth
- pH of wastewater
- Air pressure drop
- Blower type.
- Packing material
- Packing spacing
- Water temperature.
- Ammonia concentration of the wastewater.
4/3/21
Phosporous recovery:-
Struvite:-
Struvite (MgNH4PO4· 6H2O), a proven slow-releasing fertilizer that does not burn the roots of crops, can be produced from urine. With the presence of high PO4 and NH4+ in the urine, and externally added magnesium (Mg2+), struvite crystals can be obtained via precipitation in a 1:1:1 molar ratio. Studies on the thermodynamics and influencing factors of struvite precipitation from human urine have been covered in lab-scale. With the production of struvite, 90% PO4 3- but merely 3% NH4+ present in the urine can be recovered due to the proportionally higher ammonium than phosphate concentrations, and thus subsequent steps for NH4+ removal are required. Maurer et al. (2006) summarized the different NH4 + recovery methods from urine but these treatment processes rarely go beyond laboratory scale. Similarly, literature studies on pilot or full-scale demonstrations for struvite recovery from urine are very sparse. implemented struvite reactors to study the feasibility of recovering struvite from a small village in Nepal aimed for low-cost installation and operations.
8/3/21
DOE for Nitrification:-
| Factors | |||
| pH | 7 | 7 | 7 |
| C:N ratio | 1:4 | 1:8 | – |
| Dilution | 1:2 | 1:8 | 1:4 |
| pH | C:N Ratio | Dilution |
| 7 | 1:4 | 1:2 |
| 7 | 1:8 | 1:2 |
| 7 | 1:4 | 1:8 |
| 7 | 1:8 | 1:8 |
| 7 | 1:4 | 1:4 |
| 7 | 1:8 | 1:4 |
This is a Full factorial DOE for 3 factors viz. pH, C:N ratio and an Dilution factor. Therefore as You can see With number of factors summed down to 3 and with a two level factorial 6 experiments can be performed at the most.
9/3/21
Experiment Design:-
| Experiment 1 | |
| 10 Ltr | Dilution 1:1 |
| 5 ltr water | 5 ltr urine |
| 5 ltr MBBR | |
| C:N=1:4 | |
| CaCO3 till pH 7 | 15g |
| Experiment 2 | |
| 10Ltr | Dilution 1:1 |
| 5 ltr water | 5 ltr urine |
| 5 ltr MBBR | |
| C:N=1:8 | |
| CaCO3 till pH 7 | 15g |
| Experiment 3 | |
| 10 Ltr | Dilution 1:4 |
| 8.25 ltr water | 1.25 ltr urine |
| 5 ltr MBBR | |
| C:N=1:4 | |
| CaCO3 till pH 7 | 10g |
| Experiment 4 | |
| 10 Ltr | Dilution 1:4 |
| 8.25 ltr water | 1.25 ltr urine |
| 5 ltr MBBR | |
| C:N=1:8 | |
| CaCO3 till pH 7 | 10g |
| Experiment 5 | |
| 10 Ltr | Dilution 1:2 |
| 7.5 ltr water | 2.5 ltr urine |
| 5 ltr MBBR | |
| C:N=1:4 | |
| CaCO3 till pH 7 | 12g |
| Experiment 6 | |
| 10 Ltr | Dilution 1:2 |
| 7.5 ltr water | 2.5 ltr urine |
| 5 ltr MBBR | |
| C:N=1:8 | |
| CaCO3 till pH 7 | 12g |

All experiments were covered with shade nets so that there is no evaporation of urine and every experiment was given aeration via a air pump of specifications (2W, 2l/Min). In each experiment 1 Litre of BARC culture was added.
26/3/21
Results:-
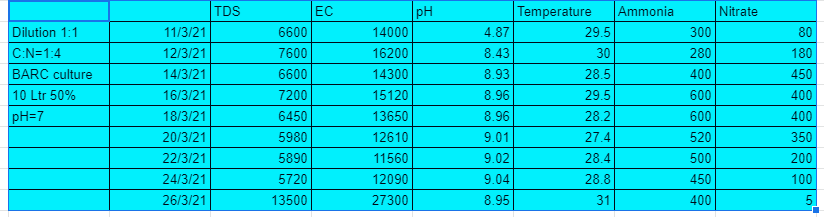
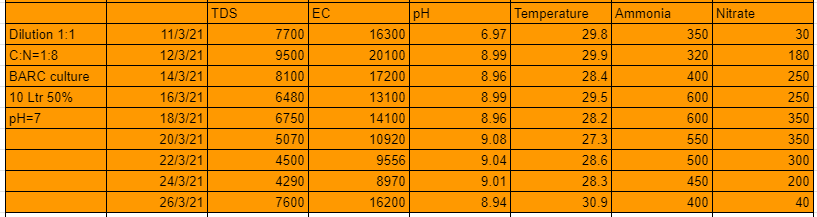
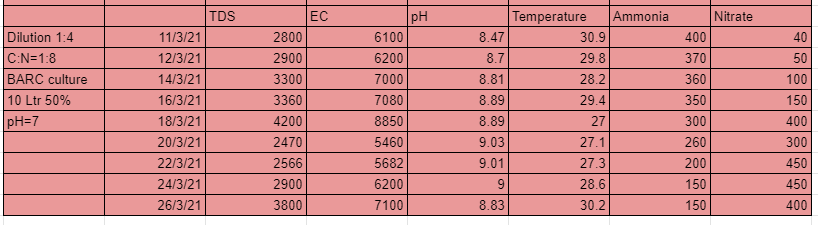


As seen from the table above the 1 st system that is 1:1 dilution and with C:N ratio=1:4 performed well in the first 10 days but after that the nitrates suddenly started falling and the pH started to grow substantially. A maximum of 400 ppm of nitrates were developed from the system.
The 2nd system that is 1:1 dilution and C:N ratio 1:8 performed well in the first 10 days but after that the nitrates suddenly started falling and the pH started to grow substantially. A maximum of 350 ppm of nitrates were developed from the system.
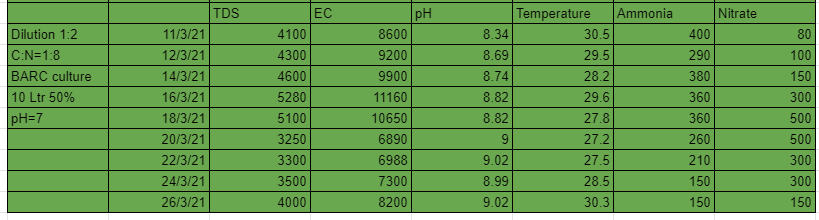
The 3rd system that is 1:2 dilution and C:N ratio 1:4 performed well in the first 12 days but after that the nitrates suddenly started falling and the pH started to grow substantially. A maximum of 220 ppm of nitrates were developed from the system.
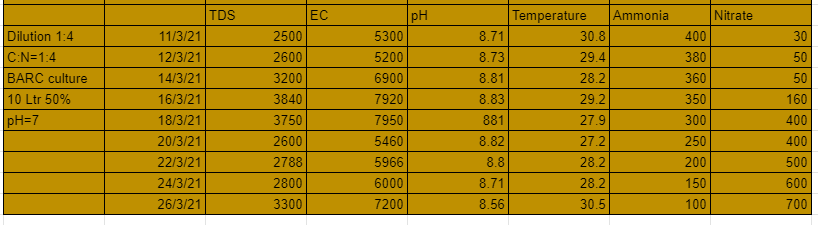
The 4th system that is 1:2 dilution and C:N ratio 1:8 performed well in the first 10 days but after that the nitrates suddenly started falling and the pH started to grow substantially. A maximum of 500 ppm of nitrates were developed from the system. This shows with lesser amount of carbon still greater nitrates could be generated.
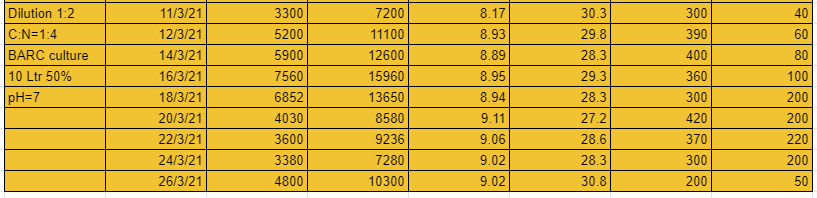
The 5th system that is 1:4 dilution and C:N ratio 1:4 was the best of the lot. Nitrates developed upto 700ppm. You could also see the pH droping and the ammonia levels too.
The 6th system that is 1:4 dilution and C:N ratio 1:8 also performed very well with nitrates formation upto 450 ppm. You could also see the pH droping and the ammonia levels too.
From these experiments we conclude that with dilution ratio 1:4 and C:N ratio 1:4 we can generate tremendous amount of nitrates. The lesser dilution systems can grow nitrates upto a certain level only, this could be due to high TDS and EC cause by the amount of salt in the urine.
12/3/21
Composting of Sugarcane strands and Rice husk :-
The farmers in India or in and around pune region burn the sugarcane strands and the rice husk as well as wheat strands as they are have very slow or no decomposing capacity. Therefore this residue is burnt off and a lot of CO2 is emitted from such burnings. Which in turn leads to pollution and emission of a greenhouse gas. Therefore we here at Vigyan ashram thought of solving this problem via composting. Cause these materials have a huge amount Carbon content inside them and urine consisting of a whole lot of nitrogen in it, adjusting proper C:N ratios a nice composting material can be made.
So firstly we checked the water holding capacity of these two materials i.e sugarcane strands, rice husk. So we took 100 g each of strands and husk on a sieve and 1 litre urine was poured over each of the material and a bucket was kept below so that the quantity of urine absorbed by both these materials could be found out. Our results show that 100 g of sugarcane strands absorbs 300 ml of urine and 100 g rice husk absorbs 350ml of urine.
Therefore now we setup an experiment for 2litre of urine. Where we took a 2 ltr of urine in an 20 l bucket and then we added these sugarcane strands and rice husk in two separate buckets. We found out that 650g of sugarcane strands could absorb 2 ltr of urine and 1067g of rice husk could absorb 2 ltr of urine.
15/3/21
| Usacha Pachat (Sugarcane strands) | |
| Start weight | 650g |
| After urine Addidtion | 1406g |
| Start Volume | 7 Ltr |
| After urine addition | 8.8 Ltr |
| % increase in weight(dry/wet basis) | 116 |
| % increase in Volume | 20 |
| Bhatacha Tus (Rice husk) | |
| Start weight | 1067g |
| After urine Addidtion | 2760g |
| Start Volume | 9.1 Ltr |
| After urine addition | 10.25 Ltr |
| % increase in weight(dry/wet Basis) | 158 |
| % increase in Volume | 11 |
24/3/21
Hydroponics:-
Generating gold from waste was always and will be and great idea. We tried doing something on similar grounds with the medium being hydroponics. We reached to a conclusion that urine contains more than enough nutrients for palak, lettuce and tomato to grow we will see exactly how.
| Nutrients | Palak Nutrients in mg / (100g) | Lettuce nutrients in mg / (100g) | Urine nutrients in mg/ 100 ml urine | Urine nutrients in mg/ 1Litre urine |
| Calcium | 99 | 35 | 15 | 150 |
| Iron | 2.71 | 2 | 0.0205 | 0.205 |
| Magnesium | 79 | 13 | 10 | 100 |
| Phosphorous | 49 | 26 | 120(phosphate) | 382.9(phosphorous) |
| Potassium | 558 | 238 | 150 | 1500 |
| Sodium | 79 | 5 | 600 | 6000 |
| Zinc | 0.53 | 0.2 | 0.011 | 0.11 |
| Copper | 0.13 | – | 0.0155 | 0.155 |
| Manganese | 0.897 | 0.179 | 0 | 0 |
| Selenium | 1 | – | – | 0 |
| Nitrogen | 490 | 144 | 920 | 9200 |
| Chloride | – | – | 600 | 6000 |
| Sulfur | 0.0042 | 0.0058 | 180 | 1800 |
As you can see urine fulfills all the essential nutrients that palak(spinach)and lettuce require. But the problem arises now of the sat content of urine. urine contains a lot of salts. Approximately 6g/L. This leads to tremendous amount of EC which spinach may be able to sustain according to literature.
| Palak Salt tolerating capacity | 9.4 | dS/m |
| Urine EC via salt | 7.5 | dS/m |
| Nitrogen content in 100g palak | 0.49 | g |
| Nitrogen content in 100g urine | 0.92 | g |
| Nitrogen content in 1000g urine | 9.2 | g |
| Palak obtainable from 1000g urine with 9.2 g nitrogen | 1877.55102 | g |
| Potassium from 100g palak | 0.558 | g |
| Potassium from 100g urine | 0.15 | g |
| Potassium from 1000 g urine | 1.5 | g |
| Palak obtainable from 1000g urine with 1.5 g potassium | 268.8172043 | g |
| Phosphorous from 100g palak | 0.049 | g |
| Phosphorous from 100g urine | 0.0382 | g |
| Phosphorous from 1000 g urine | 0.382 | g |
| Palak obtainable from 1000g urine with 3.82 g phosphorous | 779.5918367 | g |
| Magnesium from 100g palak | 0.079 | g |
| Magnesium from 100g urine | 0.01 | g |
| Magnesium from 1000 g urine | 0.1 | g |
| Palak obtainable from 1000g urine with 0.1 g magnesium | 126.5822785 | g |
| Calcium from 100g palak | 0.099 | g |
| Calcium from 100g urine | 0.015 | g |
| Calcium from 1000 g urine | 0.15 | g |
| Palak obtainable from 1000g urine with 0.15 g calcium | 151.5151515 | g |
| Additional Potassium required to obtain 1.87 kg palak from 1 ltr urine | 8.976734694 | g |
| Additional Phosphorous required to obtain 1.87 kg palak from 1 ltr urine | 0.538 | g |
| Additional Magnesium required to obtain 1.87 kg palak from 1 ltr urine | 1.383265306 | g |
| Additional Calcium required to obtain 1.87 kg palak from 1 ltr urine | 1.70877551 | g |
| Cost of mono potassium phosphate | 95 | rs/kg |
| Cost of di potassium phosphate | 90 | rs/kg |
| Cost of potassium suplfate | 25 | rs/kg |
| Cost of potassium carbonate | 80 | rs/kg |
| Potassium content from mono potassium phosphate (per 100g) | 28 | g |
| Potassium content from Di potassium phosphate (per 100g) | 44 | g |
| Potassium content from potassium sulfate (per 100g) | 44 | g |
| Potassium content from potassium carbonate (per 100g) | 56 | g |
| Cost of Phosphoric acid | 65 | rs/kg |
| Phosphorous content from phosphoric acid (per 100g) | 30 | g |
| Cost of calcium carbonate | 10 | rs/kg |
| Calcium content from calcium carbonate (per 100g) | 40 | g |
| Cost of 44 g of potassium from potassium sulfate | 3.2 | rs |
| Cost of 8.97 g of potassium required to grow 1.87 kg palak per litre urine | 0.5745110204 | rs |
| Cost of 56 g of potassium from potassium carbonate | 8 | rs |
| Cost of 8.97 g of potassium required to grow 1.87 kg palak per litre urine | 1.282390671 | rs |
| Cost of 44 g of potassium from dipotassium phosphate | 9 | rs |
| Cost of 8.97 g of potassium required to grow 1.87 kg palak per litre urine | 1.836150278 | rs |
| Cost of 32.5 g of potassium from mono potassium phosphate | 9.5 | rs |
| Cost of 8.97 g of potassium required to grow 1.87 kg palak per litre urine | 2.623968603 | rs |
| Cost of 17 g phosphorous from di potassium phosphate | 9 | rs |
| Cost of 0.538 g of phosphorous required to grow 1.87 kg palak per litre urine | 0.2848235294 | rs |
| Cost of 22 g phosphorous from mono potassium phosphate | 9.5 | rs |
| Cost of 0.538 g of phosphorous required to grow 1.87 kg palak per litre urine | 0.2323181818 | rs |
| Cost of 31 g phosphorous from phosphoric acid | 6.5 | rs |
| Cost of 0.538 g of phosphorous required to grow 1.87 kg palak per litre urine | 0.1128064516 | rs |
| Cost of 40 g calcium from calcium carbonate | 1 | rs |
| Cost of 1.7 g of calcium required to grow 1.87 kg palak per litre urine | 0.04271938776 | rs |
| Nitrogen | Phosphorous | Potassium | Calcium | Magnesium | |
| 100g Palak(g) | 0.49 | 0.049 | 0.558 | 0.099 | 0.079 |
| 100g Urine(g) | 0.92 | 0.0382 | 0.15 | 0.015 | 0.01 |
| 1000g Urine(g) | 9.2 | 0.382 | 1.5 | 0.15 | 0.1 |
| Palak from 1000 g urine per nutrient | 1877.55102 | 779.5918367 | 268.8172043 | 151.5151515 | 126.5822785 |
| Additional Potassium required to obtain 1.87 kg palak from 1 ltr urine | 8.976734694 | g |
| Additional Phosphorous required to obtain 1.87 kg palak from 1 ltr urine | 0.538 | g |
| Additional Magnesium required to obtain 1.87 kg palak from 1 ltr urine | 1.383265306 | g |
| Additional Calcium required to obtain 1.87 kg palak from 1 ltr urine | 1.70877551 | g |
| Fertilizer | Cost(rs/kg) | Cost(rs/100g) |
| Mono potassium phosphate | 95 | 9.5 |
| Dipotassium phosphate | 90 | 9 |
| Potassium suplfate | 32 | 3.2 |
| Potassium carbonate | 80 | 8 |
| Phosphoric acid | 65 | 6.5 |
| Calcium carbonate | 10 | 1 |
| Potassium content from mono potassium phosphate (per 100g) | 28 | g |
| Potassium content from Di potassium phosphate (per 100g) | 44 | g |
| Potassium content from potassium sulfate (per 100g) | 44 | g |
| Potassium content from potassium carbonate (per 100g) | 56 | g |
| Phosphorous content from phosphoric acid (per 100g) | 30 | g |
| Phosphorous content from mono potassium phosphate (per 100g) | 22 | g |
| Phosphorous content from Dipotassium phosphate (per 100g) | 17 | g |
| Calcium content from calcium carbonate (per 100g) | 40 | g |
| Nutrient | Fertilizer | Cost(rs)/per litre urine |
| Potassium | Potassium Sulfate | 0.6528534323 |
| Potassium carbonate | 1.282390671 | |
| Mono Potassium phosphate | 3.045677843 | |
| Dipotassium phosphate | 1.836150278 | |
| Phosphorous | Mono Potassium phosphate | 0.2323181818 |
| Dipotassium phosphate | 0.2848235294 | |
| Phosphoric acid | 0.1165666667 | |
| Calcium | Calcium carbonate | 0.04271938776 |
| Set 1 | |
| Potassium sulphate | 0.6528534323 |
| Phosphoric acid | 0.1165666667 |
| Calcium carbonate | 0.04271938776 |
| Total cost to produce 1.87 kg Spinach | 0.8121394867 |
| Set 2 | |
| Dipotassium phosphate | 1.836150278 |
| Calcium carbonate | 0.04271938776 |
| Total cost to produce 1.87 kg Spinach | 1.878869666 |
Previous work done at vigyan ashram suggests that for producing 1 kg palak requires 1.06 rs. Therefore for producing 1.87 kg palak the system requires 1.96 rs. But with the sytem proposed above, to produce 1.87 kg palak requires only 0.81 rs. A huge boost to the system. So we tried implementing the sytem.
25/3/21
Experimentation:-
The 5 th system from the nitrification experiments was taken into account for preparation of the hydroponics system.
| Dilution1:4 | TDS | 2900 |
| C:N=1:4 | EC | 6200 |
| 10Ltr system. | pH | 8.52 |
| Temperature | 29.3 | |
| Ammonia | 150 | |
| Nitrates | 700 |
| Normal | TDS | 167 |
| Tap Water | EC | 356 |
| pH | 8.56 | |
| Temperature | 30.2 | |
| Ammonia | 0 | |
| Nitrates | 0 |
A 40 lit system was setup with 3 Litre urine and 37 lit water. to it BARC culture was added. And to cover the amount of potassium calcium and phosphorous respective amounts of potassium sulphate, calcium carbonate and phosphoric acid were added to the system.
| After dilution | TDS | 1055 |
| EC | 2254 | |
| 40Ltr system. | pH | 6.33 |
| Temperature | 30.3 | |
| Ammonia | 100 | |
| Nitrates | 600 |


As you can the system was adjusted to 2250 and pH was maintained below 7. Now we checked the system working over the next few days. Here’s what we found.

As you can see al the spinach which was growing properly couldn’t grow in the system setup by us. The possible reasons could be due to heavy EC that the spinach couldn’t sustain. The second reason could be due to sun stroke. the temperatures reached 38 C on the first day. the third and the most important reason could be the excessive nitrates inside the system. The palak received a heavy dose of 600 ppm nitrates on the first day itself. And this could be the major reason for the palak not growing.
So we decided to drop down the EC and the amount of nitrates in the system. Therefore a new system was setup with 2 litre urine and dilution with tap water to 40 litre.
| Urine | TDS | 18100 |
| EC | 38500 | |
| pH | 1.51 | |
| Temperature | 28.8 | |
| Ammonia | 500 | |
| Nitrates | 10 |
| Normal | TDS | 167 |
| Tap Water | EC | 356 |
| pH | 8.56 | |
| Temperature | 30.2 | |
| Ammonia | 0 | |
| Nitrates | 0 |
| After dilution | TDS | 594 |
| EC | 1263 | |
| 40Ltr system. | pH | 5.11 |
| Temperature | 27.9 | |
| Ammonia | 130 | |
| Nitrates | 5 |
As you can see that the system EC was brought down to 1200 mS/cm and the pH was dropped down to 5. Therefore to increase the pH was necessary. hence we added calcium carbonate to the system.
Ammonia Stripping Experiments:-
DOE for Stripping experiments:-
| Factors | Low | High |
| pH | 9 | 12 |
| Temperature(0C) | 30 | 40 |
| Flow Rate(L/Min) | 2 | 3 |
| Packing of column(%) | 50 | 60 |
| pH | Temperature | Flow rate | Packing of Column |
| 9 | 30 | 2 | 50 |
| 12 | 30 | 2 | 50 |
| 9 | 40 | 2 | 50 |
| 12 | 40 | 2 | 50 |
| 9 | 30 | 3 | 50 |
| 12 | 30 | 3 | 50 |
| 9 | 40 | 3 | 50 |
| 12 | 40 | 3 | 50 |
| 9 | 30 | 2 | 60 |
| 12 | 30 | 2 | 60 |
| 9 | 40 | 2 | 60 |
| 12 | 40 | 2 | 60 |
| 9 | 30 | 3 | 60 |
| 12 | 30 | 3 | 60 |
| 9 | 40 | 3 | 60 |
| 12 | 40 | 3 | 60 |
| Experiment 1 |
| 1 ltr urine |
| pH=9 |
| Temperaure 30 |
| Flow rate 2L/Min |
| packing 50% |
| Experiment 2 |
| 1 ltr urine |
| pH=12 |
| Temperaure 30 |
| Flow rate 2L/Min |
| packing 50% |
| Experiment 3 |
| 1 ltr urine |
| pH=9 |
| Temperaure 40 |
| Flow rate 2L/Min |
| packing 50% |
| Experiment 4 |
| 1 ltr urine |
| pH=12 |
| Temperaure 40 |
| Flow rate 2L/Min |
| packing 50% |
| Experiment 5 |
| 1 ltr urine |
| pH=9 |
| Temperaure 30 |
| Flow rate 3L/Min |
| packing 50% |
| Experiment 6 |
| 1 ltr urine |
| pH=12 |
| Temperaure 30 |
| Flow rate 3L/Min |
| packing 50% |
| Experiment 7 |
| 1 ltr urine |
| pH=9 |
| Temperaure 40 |
| Flow rate 3L/Min |
| packing 50% |
| Experiment 8 |
| 1 ltr urine |
| pH=12 |
| Temperaure 40 |
| Flow rate 3L/Min |
| packing 50% |
| Experiment 9 |
| 1 ltr urine |
| pH=9 |
| Temperaure 30 |
| Flow rate 2L/Min |
| packing 60% |
| Experiment 10 |
| 10 ltr urine |
| pH=12 |
| Temperaure 30 |
| Flow rate 2L/Min |
| packing 60% |
| Experiment 11 |
| 10 ltr urine |
| pH=9 |
| Temperaure 40 |
| Flow rate 2L/Min |
| packing 60% |
| Experiment 12 |
| 10 ltr urine |
| pH=12 |
| Temperaure 40 |
| Flow rate 2L/Min |
| packing 60% |
| Experiment 13 |
| 1 ltr urine |
| pH=9 |
| Temperaure 30 |
| Flow rate 3L/Min |
| packing 60% |
| Experiment 14 |
| 1 ltr urine |
| pH=12 |
| Temperaure 30 |
| Flow rate 3L/Min |
| packing 60% |
| Experiment 15 |
| 1 ltr urine |
| pH=9 |
| Temperaure 40 |
| Flow rate 3L/Min |
| packing 60% |
| Experiment 16 |
| 1 ltr urine |
| pH=12 |
| Temperaure 40 |
| Flow rate 3L/Min |
| packing 60% |
To start with with setup a blank system or a control system as follows. A 2 Litre system was setup at vigyan ashram soil lab. With a reactor made up of plastic. Diffuser was placed inside this reactor for effective mass transfer and a pipe was given from inside the system to a volumetric flask containing 0.5 m sulfuric acid.

Nitrification Experiments:-
1/4/2021
| Factors | ||
| C:N ratio | 1:2 | 1:8 |
| Dilution | 01:10 | 1:0.5 |
So we had achieved good results from the previous nitrification experiments, but there was a need of optimization i.e time needed the for the complete nitrification should be reduced, the quantity of nitrates should be threshold. So we designed 4 new experiments with 2 factors C:N ratio and Dilution ratio.
| Experiment 1 | |
| 10 Ltr | Dilution 1:10 |
| 10 ltr water | 1 ltr urine |
| 5 ltr MBBR | |
| C:N=1:2 | |
| CaCO3 till pH 7 |
| Experiment 2 | |
| 10 Ltr | Dilution 1:10 |
| 10 ltr water | 1 ltr urine |
| 5 ltr MBBR | |
| C:N=1:8 | |
| CaCO3 till pH 7 |
| Experiment 3 | |
| 10 Ltr | Dilution 1:0.5 |
| 10 ltr water | 1 ltr urine |
| 5 ltr MBBR | |
| C:N=1:2 | |
| CaCO3 till pH 7 |
| Experiment 4 | |
| 10 Ltr | Dilution 1:0.5 |
| 10 ltr water | 1 ltr urine |
| 5 ltr MBBR | |
| C:N=1:8 | |
| CaCO3 till pH 7 |

All experiments were covered with shade nets so that there is no evaporation of urine and every experiment was given aeration via a air pump of specifications (2W, 2l/Min). In each experiment 1 Litre of BARC culture was added.
3/4/2021
Duckweed growth trials with Urine:-
The previous work that I had Performed at vigyan ashram was
1) Nitrates generated from the process achieved an yield of upto 86%
2) The culture developed during the course of the experiments was used for biofloc for fish farming.
3) Ammonia was brought down to 0.25ppm inside the biofloc from 12 ppm.
4) On the whole the entire cycle of hydroponics and aquaponics now looks economically feasible.
So now there’s something new that we found in the aquaponics system. That duckweed a potential nitrogen source for the fish has been taken in plenty by the fish inside the aquaponics plant, more than the normal feed that is given to them. So on similar grounds we tried to build a new system where we would generate nitrates with urine as a nutrient source. And this would be in turn would be given to the aquaponics plant. So here’s what the whole system looks like. Fish i the aquaponics plant would generate ammonia. this ammonia would be brought to a bed where nitrates would be generated and where duckweed would grow. This duckweed would be then fed to the fish. Which complete a circular economy with no additional source nutrient required. And for additional duckweed we would use urine as a source nutrient. So here’s what we put forth.


So a bed of 500 litre capacity and a tank of 200 litre capacity were chosen as potential reactors for the system. The bed was given 100 litre of urine and rest was filled with water. The tank was kept for nitrification, with MBBR for bacterial growth and aeration via an air pump. A submersible pump was kept inside the system so that the water from the bed goes to the tank and then in turn treats the water(for nitrification). And a cyphen was given so that the remainder of water from the system moves out to the bed.
As we can see there was duckweed growth in the system on the first day itself. But the very next day they turned to a greenish yellow color meaning the cells were dead. The possible reasons could be,
- Heavy EC of urine water
- No sunlight for growth
- Amount of nitrates and ammonia in the system.
From this we could conclude that the growth trials need to optimized with optimum amount of ammonia and the other nutrients required for its growth.
Composting trials:-
5/4/2021
So as we can see we setup a system where we setup a rack system. The first compartment had 1 kg of rice husk and the second compartment had 1 kg of Sugarcane strands. Now poured the right of urine and water for the system to develop and Culture of about 300 ml everyday. We placed a submersible pump inside the blue tray where the left over urine water was collected. Now a timer was placed for the pump to spray water from time to time. The time being 1 min spraying for every hour.

As we can see the earlier trials that we had done, now have started turning black after 30 days. Which is a good sign of composting.
10/4/21
Spinach And Duckweed:-
| DM 4.7 % | Urine(mg/l) | |
| Nutrients | Duckweed(kg/ha/yr) | |
| Calcium | 6000 | 150 |
| Iron | 800 | 0.205 |
| Magnesium | 800 | 100 |
| Phosphorous | 800 | 382.9 |
| Potassium | 2520 | 1500 |
| Sodium | 390 | 6000 |
| Zinc | 7 | 0.11 |
| Copper | 1 | 0.155 |
| Manganese | 90 | 0 |
| Selenium | 1 | – |
| Nitrogen | 6100 | 9200 |
| Chloride | 1000 | 6000 |
| Sulfur | 650 | 1800 |
| DM 4.7 % | ||
| Crude protein | 38.6 | % |
| Ether extract | 9.8 | % |
| NFE | 8.6 | % |
| Fibre | 18.7 | % |
| Ash | 19 | % |
| Ca | 0.7 | % |
| P | 0.6 | % |
| K | 4.3 | % |
| Fe | 0.3 | % |
| Mn(mg/kg) | 75 | |
| Cu(mg/Kg) | 20 | |
| Carotene(mg/kg) | 1025 |
As we can see from the tables urine fulfills almost every nutrient that the duckweed requires for its growth. All the nutrients including N, P, K, Ca, Mg are fulfilled by the nutrients from 1 Litre Urine.
12/4/21
Comparison of Nutrients in Spinach and duckweed with urine:-
| Nutrient | Duckweed(g/100g) | Urine(g/Kg) | Duckweed obtainable per litre urine.(g) |
| Calcium | 0.6 | 0.15 | 25 |
| Iron | 0.023 | 0.000205 | 0.8913043478 |
| Magnesium | 0.454 | 0.1 | 22.02643172 |
| Phosphorous | 0.704 | 0.382 | 54.26136364 |
| Potassium | 2.3 | 1.5 | 65.2173913 |
| Sodium | 0.025 | 6 | 24000 |
| Zinc | 0.0053 | 0.00011 | 2.075471698 |
| Copper | 0.000352 | 0.000155 | 44.03409091 |
| Manganese | 0.075 | 0 | 0 |
| Selenium | 0.003 | 0 | 0 |
| Nitrogen | 3.2 | 9.2 | 287.5 |
| Chloride | 0.025 | 6 | 24000 |
| Sulfur | 0.17 | 1.8 | 1058.823529 |
| Nutrient | Duckweed(g/100g) | Palak(g/100g) | Urine(g/Kg) |
| Calcium | 0.6 | 0.099 | 0.15 |
| Iron | 0.023 | 0.00271 | 0.000205 |
| Magnesium | 0.454 | 0.079 | 0.1 |
| Phosphorous | 0.704 | 0.049 | 0.382 |
| Potassium | 2.3 | 0.558 | 1.5 |
| Sodium | 0.025 | 0.079 | 6 |
| Zinc | 0.0053 | 0.00053 | 0.00011 |
| Copper | 0.000352 | 0.00013 | 0.000155 |
| Manganese | 0.075 | 0.000897 | 0 |
| Selenium | 0.003 | 0.001 | 0 |
| Nitrogen | 3.2 | 0.49 | 9.2 |
| Chloride | 0.025 | – | 6 |
| Sulfur | 0.17 | 0.0000042 | 1.8 |
As we can see from the table that duckweed requires more amount of calcium, magnesium, iron, and phosphorous. Other than that urine fulfills almost every nutrient required for its growth.
| Palak obtainable from 1000g urine(g) | Duckweed obtainable from 1000g urine(g) | |
| Nitrogen | 1877.5 | 287.5 |
| Phosphorous | 779.59 | 54.26 |
| Potassium | 268.81 | 65.21 |
| Calcium | 151.51 | 25 |
| Magnesium | 126.58 | 22.02 |
The above table shows us the amount of spinach or duckweed that could be grown from the respective nutrients obtained and utilized from urine. So now we decided to potassium as a limiting nutrient and check whether the system of Spinach and duckweed becomes feasible economically. The economic feasibility parameter comes into picture because when nitrogen is used as limiting nutrient it requires a lot of dilution which in turn means larger surface area for growth. And therefore land charges with more amount of aeration.
| Cost saved for N if we use urine | 0.2 | rs |
| Cost saved for P if we use urine | 0.82 | rs |
| Cost saved for K if we use urine | 1.09 | rs |
| If Potassium is used as a limiting nutrient palak generation from K content in urine | 268.8172043 | g |
| Amount of palak generated from Kitchen hydroponics unit per day | 142.8571429 | g |
| Area of kitchen hydroponics unit | 2 | m2 |
| Amount of urine generated daily at vigyan ashram | 60 | Lit |
| Amount of palak to be generated from K being a limiting nutrient | 16129.03226 | g |
| Surface area required for growing palak | 225.8064516 | m2 |
So as seen from the table 60 litre of human would be generated for a 30 person urinal at vigyan ashram considering each person would donate roughly 2 lit of urine everyday. Also if urine is used as a fertilizer we save around 2 rupees every kg spinach. If potassium is used as a limiting nutrient spinach generated should be 268 g per day. Amount of Spinach generated from existing hydroponics system per day is 142 g. therefore amount of spinach generated from a 30 person urinal at vigyan ashram should be around 16 kg spinach. And the amount of surface area required is 225 m2.
| Area of Duckweed bed | 4 | m2 |
| Amount of duckweed grown from duckweed bed per day | 500 | g |
| Amount of urine generated daily at vigyan ashram | 60 | Lit |
| Amount of Duckweed to be generated from K being a limiting nutrient | 3913.043478 | g | ||
| Amount of Duckweed to be generated from N being a limiting nutrient | 17250 | g | ||
| Amount of Duckweed to be generated from P being a limiting nutrient | 3255.681818 | g | ||
| Surface area required for growing Duckweed for K limiting | 31.30434783 | m2 | ||
| Surface area required for growing Duckweed for K limiting for same amount as of 16 kg palak | 128 | m2 | ||
| Time required for same amount of duckweed as palak | 5.333333333 | Days | ||
| Surface area required for growing Duckweed for N limiting | 138 | m2 | ||
| Surface area required for growing Duckweed for P limiting | 26.04545455 | m2 | ||
| Surface area required for growing Duckweed for N limiting for same amount as of 16 kg palak | 128 | m2 | ||
| Surface area required for growing Duckweed for P limiting for same amount as of 16 kg palak | 128 | m2 |
As we can see from the table amount of duckweed to be obtained from K being a limiting nutrient is approximately 4 kg. Surface area required for growing 4 kg duckweed is 31 m2. but if compared to the amount of spinach i.e 16 kg the surface area required is 128 m2. So the area almost becomes half the area required for growing spinach. And it also has more nutritional value than spinach. This looks good on paper so we decided to just give a proof of concept.
19/4/21
Experimentation:-

4 new experiments were setup at vigyan ashram soil lab. dilution ratios were kept at 1:150, 1:200, 1:250, 1:300. all were 10 Litre experiments. each experiment had 50 g of duckweed in it. Starting from the left first experiment had a dilution ratio of 1:300, second experiment with 1:250 dilution ratio, third experiment with 1:200 dilution ratio, and a fourth experiment 1: 150 dilution ratio.
15/4/21
Hydroponics using Urine :-
Experimentation:-
So after a thorough research we found some interesting results and facts about hydroponics using urine. Dilution of 1:40 and 1:50 were used by some researchers to achieve optimum and good results. With pH being around 6.5 to 7.5. So we decided to check these systems at our place.
17/4/21
Composting Trials:-

19/4/21
New growth trials for Duckweed:-

A bed of size 6 ft * 4 ft was chosen for growth trials of duckweed. It had a 500 litre capacity. After a thorough research we found out that duckweed require optimum growth requires 1:200 dilution, and an ammonia levels around 60-100 mg/L. So we setup a bed of 1 :200 Dilution and growth trials were checked upon everyday.
Surface area Required for duckweed to grow:-
So a plastic container of surface area 0.112 m2 was chosen to calculate the amount duckweed that fits over this surface. So our results say that 185 g of duckweed fits in 0.112 m2 area on wet basis. But duckweed contains around 5% of Dry matter. therefore 1 m2 area would contain approximately 90 g duckweed on dry/wet basis.
21/4/21
Growth of duckweed over sack bags:-
Earlier successful trials on growing azolla over sack bags were conducted at vigyan ashram. This was done to check and increase the amount of surface area for growing azolla. and check if could grown over wet walls. So a trial with a sack bag of 46*36 cm dimension was setup at vigyan ashram. 20 g of duckweed was put on sack on wet weight basis. This is a flood and drain type system, where the timer and relay was placed for the water submersible pump. the time being 1 min of spraying and 2 hours waiting. Results were checked upon 1 week.

23/4/2021
Ash analysis of Duckweed:-
So we wanted to find what duckweed’s dry matter and its ash content. And further if could be used for bioethanol preparation.
So duckweed both wet weight and dry weight were taken for ash analysis. There samples of duckweed each 5g were put directly put in the muffle furnace at 6250C for 3 h. Then they were cooled down inside a desiccator. after cooling the ash was found white in color exactly what the doctor ordered. Now the weight of each sample was taken and ash content was found out, which is represented in the table below.
| Wet weight duckweed | |||
| Sample 1 | Sample 2 | Samle 3 | |
| Wet weight(g) | 5 | 5 | 5 |
| After heating to 650OC(g) | 0.111 | 0.08 | 0.103 |
| Ash content % | 2.22 | 1.6 | 2.06 |
| Dry weight duckweed | |||
| Sample 1 | Sample 2 | Samle 3 | |
| Weight of tray(g) | 0.765 | 0.898 | 0.816 |
| Wet weight(g) | 5.036 | 5.024 | 5.024 |
| Dry weight(g) | 0.14 | 0.125 | 0.151 |
| Dry weight % | 2.779984114 | 2.488057325 | 3.005573248 |
| After heating to 650OC(g) | 0.013 | 0.01 | 0.018 |
| Ash Content% | 9.285714286 | 8 | 11.9205298 |
As we can see the ash content on an average is 12%. As there were no methods to detect iron, zinc and some other minerals like Ca, Mg, Fe we couldn’t check what the ash actually contains.

Ceramic cups with dried duckweed 
Muffle furnace 
After heating for 3h 
Ash content
24/4/2021
Hydroponics results:-
So after a thorough research we found some interesting results and facts about hydroponics using urine. Dilution of 1:40 and 1:50 were used by some researchers to achieve optimum and good results. With pH being around 6.5 to 7.5. So we decided to check these systems at our place. To it 20.5 g of K2SO4 and 1.7 g of 65 % of phosphoric acid was added.

| TDS | EC | pH | Temp | Ammonia | Nitrate | Potassium | Sodium | |
| 17/4 | 413 | 879 | 6.73 | 29 | 20 | 10 | 74 | 11 |
| 18/4 | 528 | 1122 | 7.63 | 28.5 | 70 | 80 | 560 | 75 |
| 20/4 | 563 | 1189 | 7.16 | 27.7 | 20 | 20 | 580 | 100 |
| New water added 21/04/2021 | 590 | 1266 | 6.43 | 28.1 | 10 | 30 | 800 | 560 |
| 23/4 | 649 | 1373 | 6.88 | 28.5 | 30 | 20 | 920 | 580 |
| 25/4 | 725 | 1542 | 5.72 | 27.5 | 20 | 10 | 1060 | 620 |
| New water added 27/4/2021 | 681 | 1440 | 6.89 | 28.7 | 40 | 30 | 74 | 300 |
25/4/21
Duckweed bed Results:-
| TDS | EC | pH | Temp | Ammonia | Nitrate | Potassium | Sodium | |
| 16/4 | 378 | 799 | 8.21 | 28.6 | 50 | 30 | 30 | 760 |
| 18/4 | 394 | 844 | 8.03 | 28.5 | 20 | 50 | 40 | 820 |
| 20/4 | 298 | 634 | 7.86 | 28.2 | 15 | 30 | 40 | 830 |
| 21/4 | 294 | 630 | 7.63 | 28.8 | 5 | 30 | 30 | 840 |
| Addition of 3 Litre urine 23/04/2021 | 372 | 795 | 8.29 | 28.5 | 100 | 20 | 90 | 1020 |
| 25/4 | 357 | 760 | 7.9 | 29.4 | 20 | 30 | 106 | 1440 |
| 27/4 | 339 | 718 | 7.52 | 29.8 | 20 | 20 | 100 | 1200 |

26/4/21
Results of Duckweed experiments:-
| 1:300 | 1:250 | 1:200 | 1:150 | |
| Start weight ( wet weight) gm | 50 | 50 | 50 | 50 |
| After 1 week(wet weight) gm | 25 | 56 | 68 | 108 |