Date-07-09-2021
1 Introduction
Biogas is an environmental friendly and one of the most efficient and effective options for renewable energy among various other alternative sources . Biogas is produced by the bio-methanation process, and the effluent from the process is rich in essential nutrients that can be utilized as a very good fertilizer. Bio-methanation is the degradation of organic materials by microorganisms in the absence of oxygen. It is a multi-step biological process in which organic carbon is converted mostly to carbon dioxide and methane . Biogas can be produced from variety of substrates, such as animal manure, energy crops, industrial waste and so on. The typical reactions that occur in the anaerobic digestion process are:
Acetogenic bacteria:Cellulose C6H12O6 → 2CH3COOH + 2CO2 + 4H2
Methanogenic bacteria:CH3COOH + CH4 + CO2, CO2+H2 → CH4
Cleanergy Tech Solutions Pvt. Ltd company provide sustainable organic waste management solution. they receives some calls from farmers who having their poultry farm. And they want to manage their poultry waste with some interesting way, so we are focusing to manage this waste by using biogas which is based on poultry waste. This waste contain mostly bedding material, wasted feed and feathers, the total poultry waste (litter) is much higher.
Most of the waste materials are dumped into nearby sites, although a small portion of poultry waste is used for fish and crop production by farmers. Crude dumping of this waste is not only unattractive but also environmentally unsafe. Biogas production can be a sustainable solution to treat waste materials, and the cost of waste treatment using this process is low. Poultry waste, cow dung and other waste have been used for biogas production , but the efficiency of the gas production is low.
In this study, bio-methanation of poultry litter was studied with the co-substrates cow dung and poultry droppings. A comparative analysis using different materials was performed on biogas production.
2 Materials and methods
Date-08-09-2021
2.1 Sample collection
The poultry litter and poultry droppings were collected from poultry in Vigyan Ashram.

2.2 Analysis of Poultry waste
| Parameter | nutrient content |
| Moisture | 43.33% |
| pH | 7.9 |
| N | 3.8% |
| K | 108 ppm |
| Ca | 4.6% |
| Mg | 0.7% |
| EC | 2200 ppm |
Date-09-09-2021
2.2.1 Moisture in poultry waste
To find out moisture content in poultry waste we did LOD( Loss On Drying).
- Take one dried glass plate and weight it on weighing balance and note down it.
- Then take poultry waste in it and again weight and note it.
- And put it in oven at 105°C and take weight after every 1 hr up to weight come constant.
Weight of Plate- 41.056 gm
Initial weight of waste and plate-55.781 gm (55.781-41.056=14.725gm of waste)

| Hours | Weight |
| 0 | 55.781gm |
| 1 | 50.321 gm |
| 2 | 49.597 gm |
| 3 | 49.51gm |
| 4 | 49.45gm |
| 5 | 49.44gm |
| 6 | 49.44 gm |
Final weight of dried poultry waste = last weight – plate Weight
= 49.44 -41.056
Final weight of dried poultry waste= 8.341 gm
.
Loss On Drying = Initial weight – final weight/ Initial weight X 100
=(14.725-8.341) 100/ 14.725
Loss On Drying =43.35%
Moisture content in poultry waste is 43.35%
After drying it properly, make powder of it with the help of Mortar with Pestle. and sieve it. and pack in air tight bag for further analysis.


2.2.2 pH of poultry waste
- Take 1 g of poultry waste dried sample in beaker.
- Add 100 of distilled water, stir well for about 5 minutes and keep for half an hour.
- Again stir just before immersing the electrodes and take the pH reading by using pH meter.

The pH of poultry waste is 7.9
Date-13-09-2021
2.2.3 Nitrogen in poultry waste
Reagents :-
- Sulfuric acid , 95% , reagent grade
- Catalyst (copper sulphate and potassium sulphate ):- Add 5 g of potassium sulphate and 2 g copper sulphate
- Sodium hydroxide 50% :- Dissolve 50 g sodium hydroxide in 50 ml DW
- Sulphuric acid solution 0.5 M:- Dissolve 2.4 ml of 98% Sulphuric acid in 10 ml distilled water and make volume 100 ml .
- Hydrochloric acid 0.25 N :-Dissolve 2.17 ml of HCl in 100 ml DW
- Sodium carbonate solution :- For standardization of HCl solution
PART A :- DIGESTION
- Weigh around 1 grams of dry poultry waste powder in the digestion flask and add 2 grams of catalyst and add 20 ml sulphuric acid (95%).Add glass beads in the flask to avoid excess heating .
- Digest the content in flask for 30-40 mins until the temp raises up to 160 degree Celsius .
- Color changes from blue to colorless.
PART B :- DISTILLATION
- Add 100 ml distilled water in the digestion flask and let it cool .
- Add 50 ml 50 % sodium hydroxide solution to the flask and check pH .
- Attach the flask to the distillation unit and collect the distillate in 0.5 M Sulphuric acid solution .
BACK TITRATION :-
1.Prepare 0.5 molar NaOH by adding 2gm NaOH in 98 ml D/W .
2.Then titrate it with trapping solution in which ammonia is trapped to neutralize excess acid present in trapping solution.
PART C :- TITRATION
- Add 6-7 drops of tashiro indicator to the distillate containing flask and titrate it with 0.25M HCl solution
- End point is bluish to a slight violet colour .
CALCULATIONS :-
% NITROGEN = (ml standard acid – ml blank) x N of acid x 1.4007 / weight of sample taken
Nitrogen content in Poultry waste is – 3.8%.
2.2.4 Potassium content in poultry waste
Reagents :-
- Ammonium acetate solution:- Dissolve 77 g in 900 ml in distilled water and to adjust pH 7.0 add 3 N sodium hydroxide solution . And adjust volume up to 1 liter .
- Standard potassium chloride solution (1000ppm) :- Dissolve 1.908 g potassium chloride solution in 1 liter distilled water . We get 1000 ppm solution. , then pipette out 10 ml ,5 ml , 1 ml respectively and prepare 10 , 50 , 100ppm solution .This solutions are used for calibration of flame photometer .
Procedure :-
Preparation of test solution.
- Take 5 gm of dried powder of poultry waste in 100 ml conical flask .
- Add 25 ml Ammonium acetate solution. shake it well with the help of rotary shaker for 10 min.
- Then filter the mixture by using filter paper.
- Take 5 ml filtrate form it to second conical flask and add 40 ml D/W in it.
- solution is ready to test by Flame photometer.
determination of K by flame photometer
- Switch on flame photometer and compressor, make sure the tube is placed in D/W cuvette.
- Switch on gas, and adjust flame with pure flame that s blue coloured flame.
- And wait for 5 min.( add D/W when it get finished in cuvette).adjust zero on display with fine adjustment.
- After that place tube in cuvette having poultry waste solution.
- Note down the reading. and calculate.

Calculations :-
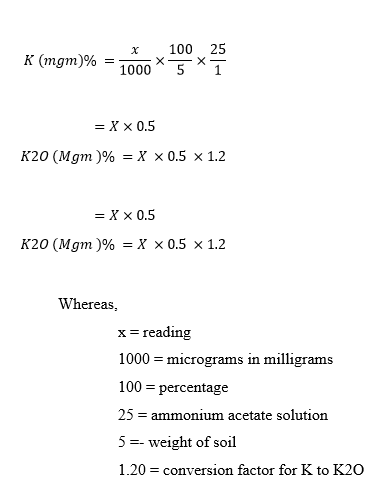
Potassium content in poultry waste is 108 ppm.
Date-15-09-2021
2.2.5 Ca and Mg in poultry waste
Reagents :-
1. Buffer Solution (NH4Cl-NH4OH) :-Dissolve 67.5 g ammonium chloride in 570 mL concentrated ammonium hydroxide, and transfer the solution to a 1-L volumetric flask, let it cool, and bring to volume with DI water.
2. Eriochrome Black Indicator:- Dissolve 0.5 g Eriochrome black with 4.5 g hydroxylamine hydrochloride in 100 mL ethyl alcohol (95%). Prepare a fresh batch every month.
3. Ethylene diamine tetra acetic Acid Solution (EDTA):- 0.01 N Dissolve 2 g ethylene diamine tetra acetic acid, and 0.05 g magnesium chloride (MgCl2) in DI water, and bring to 1-L volume with DI water.
4. Sodium Hydroxide Solution (NaOH):- 2 N Dissolve 80 g sodium hydroxide in about 800 mL DI water, transfer the solution to a 1-L volume, cool, and bring to volume with DI water.
5. Ammonium Purpurate Indicator (C8H8N6O6) :- Mix 0.5 g ammonium purpurate (Murexoid) with 100 g potassium sulfate (K2SO4).
6. Standard Stock Calcium Chloride Solution (CaCl2.2H2O):- 0.01 N Dissolve 0.5 g pure calcium carbonate (CaCO3 dried for 3 hours at 100°C), 31 UAE University in 10 mL 3 N hydrochloric acid and bring to1-L volume with DI water. This can also be prepared by dissolving 0.735 g calcium chloride dihydrate (CaCl2.2H2O) in 1-L volume with DI water.
7. Ammonium acetate solution :- Dissolve 77 grams of ammonium acetate in 80 ml of distilled water and bring volume up to 100 ml .
Procedure :-
A . Preparation of extract :-
- Weigh 50 grams of powder of poultry waste and add 200 ml of 40% ethanol to it .
- Filter the mixture and discard the filtrate and add ammonium acetate solution to it .
- Incubate the mixture overnight and use it as extract .
B. Ca Titration
- Pipette out 10-20 ml extract and dilute it with 100 ml D/W.
- Add 2-3 ml of Sodium hydroxide solution and 50 mg muroxide indictor.
- Titrate with 0.01 N EDTA , Color will changes from red to purple.
- With out using extract make a blank to minimize error.
C. Ca + Mg Titration
- Pipette out 10-20 ml extract and dilute it with 100 ml D/W.
- Add 3-5 ml buffer solution, and few drops of erichrome black indicator.
- Titrate with 0.01 N EDTA , Color will changes from red to purple.
D. Calculations
Ca or Ca + Mg (meq/L) = (V – B) × N × R × 1000 / Wt of soil sample taken
for mg = ( ca+mg ) – ca
Where:-
V = Volume of EDTA titrated for the sample (mL)
B = Blank titration volume (mL)
R = Ratio between total volume of the extract and extract volume used for titration.
N = Normality of EDTA solution
Ca and Mg in poultry waste 4.6% and 0.7% respectively.
Date-16-09-2021
2.2.6 Electric Conductivity (EC)
- EC can be measured via electrodes inserted directly into the solution.
- The solution prepared to check pH is used to find EC of poultry waste.

Electric conductivity of poultry waste is 2200 ppm.


