Introduction:
COVID-19 is the infectious disease caused by the most recently discovered coronavirus. The most common symptoms of COVID-19 are fever, tiredness, and dry cough. Some people become infected but don’t develop any symptoms and don’t feel unwell. The disease can spread from person to person through small droplets from the nose or mouth which are spread when a person with COVID-19 coughs or sneezes. These droplets land on objects and surfaces around the person. Other people then catch COVID-19 by touching these objects or surfaces, then touching their eyes, nose or mouth. (https://www.mohfw.gov.in/).
link of manual: (https://docs.google.com/document/d/1Gz9Hvzpu_HRDMkQpoK2b2qxgp-sRYf5thAwCxypr4Vw/edit?usp=sharing)
Why sodium hypochlorite solution?
Sodium hypochlorite, commonly known as bleach, is most frequently used as a disinfecting agent. It is a broad-spectrum disinfectant that is effective for the disinfection of viruses, bacteria, fungi, and mycobacterium. We can easily make it by using common salt.The Centers for Diseases Control and Prevention (CDC) and the World Health Organization recommend using a bleach solution as one way to disinfect areas contaminated with the novel coronavirus.(https://www.canr.msu.edu/news/covid-19-disinfecting-with-bleach)
Need of project:
We purchase several necessary things such as vegetables, feed of animals, groceries from the market but we can not be sure that these purchased things are disinfectant.So we had prepared a sodium hypochlorite solution which we can use at home to disinfect. We use this solution for surface sterilization and also for body disinfection. Preparation of sodium hypochlorite (NaOCl) by using common salt (NaCl) by electrolysis process.
Electrolysis Process:
● Electrolysis is a method of separating bonded elements by passing an electric current through them. An ionic compound salt is dissolved in water, so that its ions are available in the liquid.
● An electrical current is applied between a pair of inert electrodes immersed in the liquid. Each electrode attracts ions which are of the opposite charge. Therefore, positively charged ions (called cations) move towards the cathode, while negatively charged ions (termed anions) move toward the anode. The energy required to separate the ions, and cause them to gather at the respective electrodes, is provided by an electrical power supply.
Image 2: Schematic diagram of electrolysis process
Specification:
Chemicals: 1) Distilled water: 700ml, 2) Salt (NaCl) : 20gm
Technical specification:
| Input voltage | DC 15V, 2A |
| Operating temperature | 30-40°C |
| Rated power consumption | 30 watt |
| Required time | 1 hour |
Kit design:
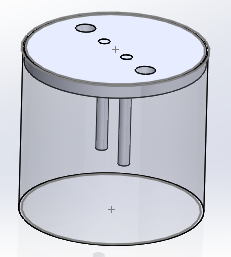
Requirement of material and specification:
| Sr. No. | Material description | Specification | Quantity |
| 1 | Glass jar | Glass or polypropylene | 1 |
| 2 | Carbon Rod https://www.indiamart.com/sharayuindustries/ | Carbon or graphite rod | 2 |
| 3 | Salt | Ionised salt | 20gm |
| 4 | Circular frame | 7cm diameter | 1 |
| 5 | Adaptor | 15V, 2A | 1 |
| 6 | Water | Distilled water | 700ml |

Reactions:
- 2H2O(l) + 2NaCl → H2(g) + Cl2(g) + 2NaOH(l)
- 2NaOH + Cl2→ NaCl + NaOCl + H2O
Calculation:
For the preparation of 0.8% NaOCl required 20 gm of NaCl in 700ml of distilled water.(According to the calculations followed by reaction.)
Faraday’s law states that the mass of substance deposited is directly proportional to the quantity of electricity.(https://www.researchgate.net/publication/233710211_On-Site_Sodium_Hypochlorite_Generation)
NaCl + H2O + 2e = NaOCl
35 lbs + 15 gal + 2.5 kWh = 0.8 % NaOCl
1587. 57 gm + 56.7812 lit + 2.5 kWh = 0.8 % NaOCl
Dividing 8 to equation
198.45 gm + 7.09765 lit + 312.5 Wh = 0.8 % NaOCl
Dividing 10 to equation
19.84 gm + 0.70965 lit + 31.25 Wh = 0.8 % NaOCl
We have to find moles of solution,
1 mol = 6.02214076×1023
For NaCl 58.5 gm
58.5 gm = 1 mole = 6.023×1023
19.84 gm = ?
x= 19.84 × 6.023 ×1023 / 58.5
| X = 0.33 moles of NaCl |
We have to find moles of electrons
1 faraday = 96500 C
1 electron = – 1.9 × 10-19
1 Faraday charge = 1 mole of e-
1 F = 6.023 × 1023 electron
= 1 mole of e-
1 A = 6.24 × 1018 electron charge / second
1A = 1C\1 second ( we use 2A current)
1A = Q / s
We have to calculate moles of charge
= 2 × 3600 × 6.024 ×1018 / 6.023 × 1023
= 0.07471 moles of electron flow
For NaOCl 74.5
74.5 gm = 1 mole
? = 0.07471 mole
X = 5.5658 gm
But we required 2 electrons
5.5658 / 2 =2.782 moles
2.782 = 700 ml
? = 1000 ml
X = 1000 × 2.782 / 700
| 3.9756% of NaOCl |
20 gm + 700 ml + 31 Whr = 0.8 % NaOCl
So, 0,8% of NaOCl required 20 gm of NaCl, 5180 C charge, 2A current, 15 volt and time will be 1 hour.
Usage guidelines:
Step 1: Take the beaker or pot and fill it up with 700ml fresh water and Add 19-20 gm of common salt in it.
Step 2: Stir it, until salt is completely dissolved in water.
Step 3: Fix carbon rod in circular frame using glue gun and connect to output supply of adapter.

Step 4: Pour the solution in a glass jar and fit a circular frame with a rod on the glass jar.

Step 5: Give the power supply to the carbon rod for one hour.

Step 6: During the reaction, the color of the solution will change and we get a final yellowish-green solution.(Note: Check pH before starting the electrolysis reaction and after the completion of the reaction. It should be between the 9-11 pH range.
Step 7: Now we can use this solution to wash vegetables, fruits etc.

Result:Solution of sodium hypochlorite has been prepared which is concluded by strong chlorine odour, yellowish-green colour and pH of solution is 9 to 11 .
Bill of material:
| Sr. No. | Material | Cost (Rs.) |
| 1 | Glass jar | 200 |
| 2 | Carbon rod | 40 |
| 3 | Adaptor | 400 |
| 4 | Circular frame | 30 |
Uses:
- To wash vegetables, fruits, feed animals.
- To clean the surface of the table,furniture etc.
- It is rarely used as an antiseptic.
- Used as a disinfectant for treating potable water (lower concentration).
- In hospitals as a high-level disinfectant.
- Bleaching agents in the textile, detergents, and paper and pulp industries.
- An oxidizing agent for organic products.
Precautions:
- Use on the outer surface of vegetables, fruits etc.
- Do not spray NaOCl on your face.
- Keep kit away when supply is on.
- Unplug the device if you are not using it.
- Keep kit away from children.
- Keep kit away from heat and high temperature
Advantages:
- Reduce the cost of disinfectant solution.
- Reduces harmful chemicals, odour, bacteria and virus from vegetables.
- Oxidizes harmful bacteria, viruses and pesticides from fruit.
- More convenient and easy
- Portable
- Easy to handle
References:
- https://www.mohfw.gov.in/
- https://www.canr.msu.edu/news/covid-19-disinfecting-with-bleach
- https://www.researchgate.net/publication/233710211_On-Site_Sodium_Hypochlorite_Generation
- https://www.kent.co.in/cooking-appliances/vegetable-fruit-cleaner/table-top-vegetable-fruit-purifier
- https://www.indiamart.com/sharayuindustries/





