10 April 2025
INTRODUCTION :- Suran, also known as Elephant Foot Yam, is an important tuber crop valued for its nutritional and medicinal properties. Conventional propagation through corms is slow and limited due to low multiplication rate and high risk of disease transmission. Plant tissue culture offers an efficient alternative for mass multiplication of disease-free, high-yielding Suran plants.
EXPLANT CAN USE :-
corm buds
shoot tip
PROPAGATION OF SURAN :-
After discussion with Dixit Sir, the plan is to do plant tissue culture of suran by taking it from the market, cutting it into equal sizes, and planting it in the soil.
We bought 2 kg of suran from the market, cut it into 4 pieces, and planted them in the soil.
19 November 2025
After nine months of cultivation, Suran (Amorphophallus paeoniifolius) tubers weighing 2 kg at the time of planting produced a total yield of 10 kg at harvest.

21December 2025
STAGES :-
1.Preparation of Explant
2. Initiation
3. Shoot Multiplication
4. Rooting
5. Hardening/Acclimatization
Table No. 1 MATERIALS :-
| Beaker | Measuring cylinder |
| Test tube | Distilled water |
| Conical flask | Culture bottle |
| Scissors | Tissue paper |
| Surgical sterile blade | Forceps |
| Autoclave bag | Sterile Distilled water |
| Scalpel | Filter paper |
| Test tube stand | Spatula |
| Petri plate | Cotton plug |
CHEMICALS FOR STERILIZATION :-
1. Luquid soap
2. Dettol
3. Sodium hypochlorite
4. Antibiotics
CHEMICALS FOR MS MEDIA :-
1. Macronutrients
2. Micronutrients
3. Vitamins
4. Iron source
5. Sucrose
6. Inositol
7. Agar
8. HCl/ NaOH
Table No. 2 PREPARATION OF MACRONUTRIENTS :-
| Macronutrients | Original Concentration | Amount of salt after multiplying original concentration by 20X, Solution Volume (250ml) |
| NH4NO3 | 1650 | 8250mg |
| KNO3 | 1900 | 9500mg |
| KH2PO4 | 170 | 850mg |
| MgSO4.7H2O | 370 | 1850mg |
| CaCl2.2H2O | 440 | 2200mg |
Table No. 3 PREPARATION OF MICRONUTRIENTS :-
| Micronutrients | Original Concentration | Amount of salt after multiplying original concentration by 100X, Solution Volume (250ml) |
| H3BO3 | 6.2 | 155mg |
| MnSO4.4H2O | 22.3 | 557.5mg |
| ZnSO4.7H2O | 8.6 | 215mg |
| Na2MoO4.2H2O | 0.25 | 6.25mg |
| CoCl2.6H2O | 0.025 | 0.625mg |
| CuSO4.5H2O | 0.025 | 0.625mg |
| KI | 0.83 | 20.75mg |
Table No. 4 PREPARATION OF VITAMINS :-
| Vitamins | Original Concentration | Original Concentration multiplying by 1000X, Final solution volume (50ml) |
| Thiamine HCl | 0.1 | 5mg |
| Pyridoxin HCl | 0.5 | 25mg |
| Nicotinic | 0.5 | 25mg |
| Glycin | 2.0 | 100mg |
Table No. 5 PREPARATION OF IRON SOURCE :-
| Iron source | Original Concentration | Original Concentration multiplying by 400X, Final solution volume (50ml) |
| FeSO4.7H2O | 27.8 | 2780mg |
| Na2EDTA.2H2O | 37.3 | 3730mg |
Table No. 6 PREPARATION OF MS MEDIA :-
| Materials | Final Volume ( 250ml ) |
| Macronutrients | 12.5ml |
| Micronutrients | 0.625ml |
| Vitamins | 0.625ml |
| Iron source | 0.250ml |
| Mesoinositol | 25mg |
| Sucrose | 7.5gm |
| Agar | 2gm |
| Distilled water | 250ml |
MESOINOSITOL:- When we prepare 1 litre ms media then we will take 100mg mesoinositol.
SUCROSE (3%):- When we prepare 1 litre ms media then we will take 30gm sucrose.PREPARATION OF
HORMONES:-
BAP Stock:– 100mg BAP in conical flask (250ml) dissolve with ethanol or 1M NaOH solution and BAP powder dissolve completely in solution and make final volume 100ml solution with Distilled water.
IBA Stock:- 100mg of IBA in conical flask (250) dissolve with ethanol or 1M NaOH solution and IBA powder dissolve completely in solution and make final volume to 100ml with Distilled water.
pH CHECK:- Prepare solution media pH range is 5.8 – 5.9 by using HCl or 1M NaOH.Agar:- Take 8 gm agar powder and add in media ,mix with glass rod then in microwave the media for 14 minutes and agar dissolve completely in media.
STERILIZATION PROCESS :-
Autoclave the used media to sterilize it before disposal. Discard the used media from the culture bottle. Soak the empty culture bottle in chromic acid (for cleaning or decontamination) for 24 hours. After 24 hours, remove the culture bottle from the chromic acid and wash it with liquid soap. Then dry it in a hot air oven at 180°C for 12 hours. Then we have wrapped glassware in autoclave bag then put in autoclave with distilled water and culture media.
Autoclave work at high pressure and temperature ( 15 psi, 121°C) for 15 to 20 minutes and kill the microorganisms. After sterilization removed glassware from sterilizer. And placed in laminar air flow then when we use it, we will turn8 on UV – ray light for 1 hour.
METHODOLOGY :-
21 December 2025
1.Select mother plants ( Shoot tips, Corm bud ) that are young shoot tips disease-free, and have high-yield and desirable traits.
2. Cut the mother plant in desirable shape and placed dry tray.
3. Wash the cut explant in running water for 15 minutes.

4. Handle the plant material with sterile gloves.
5. Then prepare total volume 300ml soap solution (30ml soap liquid and 270ml distilled water).6. Wear glove hand and wash the plant material for 5 minutes.

6. Then remove the soap solution and placed in glass beaker.
7. Cut the material desirable size with the help of knife.
8. Then soak the material in savlon solution ( 3ml savlon and 300 ml distilled water) for 15 minutes.

9. Then wash the plant material from sterile distilled water 3 to 5 time in bottle beaker.
11. Prepare 4% sodium hypochlorite solution and taking sodium hypochlorite solution into a laminar air flow and soak the plant material in sodium hypochlorite solution for 20 minutes( for 1 jar 10ml sodium hypochlorite and 250ml sterile distilled water).
12. After 20 minutes then remove plant material from sodium hypochlorite solution and wash the plant material 3 to 5 times in sterile distilled water.
13. Then cut the plant material required culture bottle size with the help of knife on sterilized brown paper.

14. Prepare antibiotic solution using Cefotaxime antibiotic( 1ml antibiotic and 200ml sterile distilled water) and soak the plant material in this solution for 30 minutes. Using only fresh antibiotic solution.
15. Then inoculate the plant material in culture medium( bottle beaker) and remove laminar air flow placed inoculate culture bottle at 24°C with photo periods of 6 to 7 hours light and 8 hours darkness for tissue culture sterile environment.
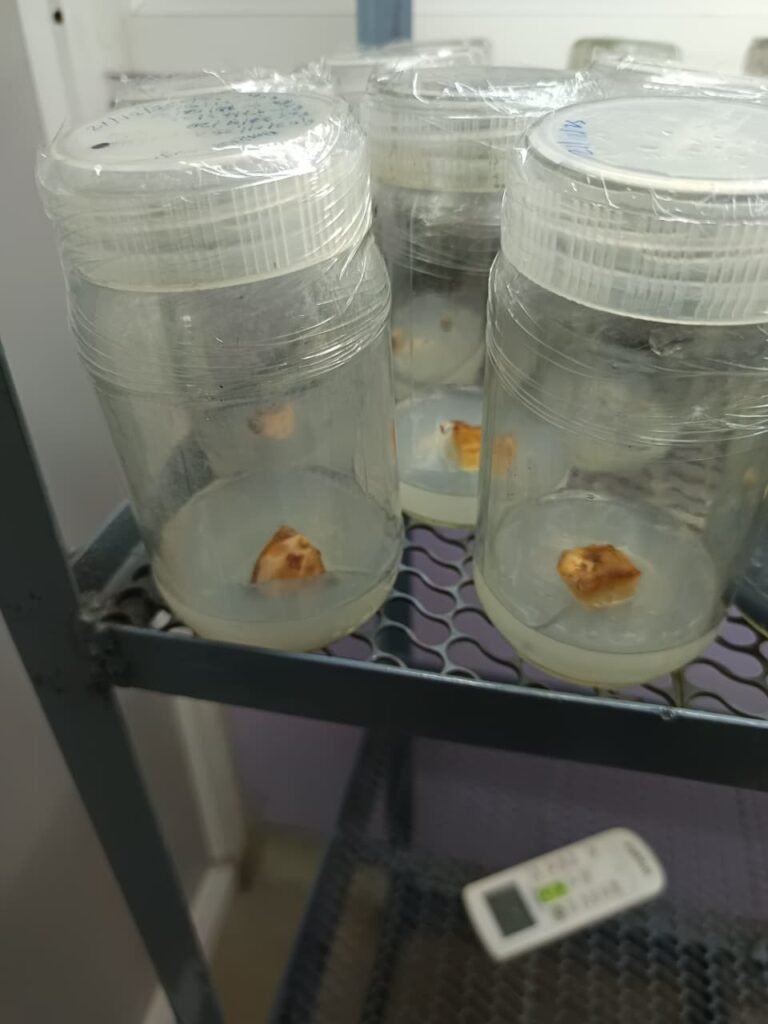

Result
Contamination appeared after 4 days.
24 December 2025
Again select a healthy suran plant and wash the explant ( shoot tip ) thoroughly. Sterilize it using a liquid soap solution, followed by soaking in an antibiotic solution for 30 minutes. After sterilization, inoculate the explant into a culture bottle under aseptic conditions.


Result
Contamination appears again after 4–5 days.
28 December 2025
I discussed the contamination issue of Suran with Dr. Dixit. He suggested preparing a 0.25% sodium hypochlorite solution and soaking the Suran in it for 30 minutes. After that, surface sterilization should be done, and the explants should be inoculated into MS medium.
Healthy yam (Suran) tubers are selected as explants and washed under running water for 15 minutes. Then, they are soaked in a 0.25% sodium hypochlorite solution for 30 minutes. After that, they are soaked in 200 ml of distilled water containing 2 g of Bavistin for 30 minutes. The tubers are then washed 3 to 4 times with distilled water. Finally, surface sterilization is done, and the explants are inoculated into MS medium.
29 December 2025
Healthy yam (Suran) tubers are selected as explants and washed under running water for 15 minutes. Then, they are soaked in a 0.25% sodium hypochlorite solution for 30 minutes. After that, they are soaked in 200 ml of distilled water containing 2 g of Bavistin for 30 minutes. The tubers are then washed 3 to 4 times with distilled water. Finally, surface sterilization is done, and the explants are inoculated into MS medium.


Result
The culture gets contaminated after one week.
6 january 2026
Suran is soaked in a sodium hypochlorite solution, then soaked in a Bavistin solution for one hour, followed by surface sterilization, and then inoculated onto MS medium.


Result :
suran got contaminated after one week.
11 January 2026
Suran is soaked in sodium hypochlorite solution, then soaked in Bavistin solution for one hour, followed by surface sterilization. The explants are then cut into 1 cm pieces and inoculated onto MS medium.


Result :
Suran showed contamination after one week.
14 January 2026
Suran is first soaked in sodium hypochlorite solution, then in Bavistin solution for one hour. After surface sterilization, the explants are cut into 1 cm pieces and placed on MS medium for inoculation.


Result :
After one week, contamination was observed in Suran.
16 January 2026
Suran is first cleaned with a sodium hypochlorite solution, then kept in a Bavistin solution for one hour. After sterilizing, the explants are cut into 1 cm pieces and placed on MS medium for growing.
23 January 2026
Suran is first soaked in sodium hypochlorite solution, then in Bavistin solution for one hour. After surface sterilization, the explants are cut into 1 cm pieces and placed on MS medium for inoculation.


Had a discussion with Dixit sir about suran. The cells were getting damaged due to the sodium hypochlorite solution, so sir suggested using monochloramine-T instead of sodium hypochlorite.
1 Fabruary 2026
First, Suran is soaked in Bavistin solution for 2 hours, then its surface is sterilized and inoculated into MS medium.


Fabruary 2026




