Introduction
Plant tissue culture broadly refers to the cultivation in vitro of all plant parts, whether a single cell, a tissue or organ under aseptic conditions. Recent progress in the field of plant tissue culture made this area as one of the most dynamic and promising experimental biology. Tissue culture technology is important for the production of disease-free, high quality planting material and the rapid production of many uniform plants.
Nutritional requirements
The composition of medium for the tissue culture is the most important key factor in the successful culture of plant cells. The medium should be accurately defined of inorganic and organic chemical additives so as to provide i) the nutrients for the survival of the plant cells, tissues and organs under culture and ii) the optimal physical condition of pH, osmotic pressure, etc.
In the culture of plant cells formulating optimum type of medium favorable for in vitro culture was achieved many years ago. The Knop’s (1865) mineral solution was the widely used medium by early investigators. Gautherat (1939) developed callus culture medium from Uspenski and Uspenskaia (1925) nutrient solution. A systematic study of mineral requirements of plant tissue and organs in culture was made by Murashige and Skoog (1962) followed by the scientists Linsmaier and Skoog (1965), Vasil and Hildebrant (1966) and Nitsch and Nitsch (1969) resulting in several media to suit particular needs.
Nutrients
A standard basal medium consists of a balanced mixture of macronutrients and micronutrients (usually salts of chlorides, nitrates, sulphates, phosphates and iodides of Ca,Mg, K, Na, Fe, Zn and B, a carbon source, vitamins, phytohormones and organic additives. Among the above mentioned nutrients some are essential and some are optional. The essential components include inorganic nutrients and organic nutrients like carbohydrates besides phytohormones and vitamins, organic additives like natural extract and liquid endosperm are optional. Inorganic salts inorganic nutrients of a plant cell culture are those required by the normal plants.The optimum concentration of each nutrient for achieving maximum growth rates varies considerably. The major elements are N, P, K, S, Mg and Ca. Other nutrients such as Co,Fe, B, Zn, Mo, Cu, I are microelements.
Macroelements
Nitrogen
Of all the mineral nutrients N plays a vital role in growth and differentiation of cultured tissues. The range of inorganic nitrogen varies from 25 mM to 60 mM according to the requirements. Nitrogen is generally supplied in the form of NH4 along with NO3. Ammonium ion as nitrogen source is usually unsuitable, probably because under such conditions the pH of the medium has a tendency to fall below 5 during culture, resulting in reduced availability of nitrogen. Cells can be grown with NH4 as the sole N source when the medium is provided with organic acids such as malate, succinate, citrate or fumerate. further, the concentration of NH4-N should not exceed 8 mM. Generally NO3-N can be used as a sole N source but often there is a beneficial effect if the media contains NH4 -N.
Phosphorus
Phosphorus is usually supplied in the form of phosphates. It is the primary buffering
constituent in tissue culture media. Phosphorus levels greater than 2mM are often inhibitory
to growth of tissues
Potassium
The optimum concentration of K needed is 20 mM. At low nitrogen concentration presence of potassium enhances the formation of somatic embryos. The medium supplemented with potassium nitrate produces more embryos than the medium with ammonium nitrate
Sulphur
Sulphur is provided in the form of sulphates. Besides, the sulphur containing amino acids like L-cysteine, L-methionine and glutathione are satisfactory sources for sulphur. Calcium and Magnesium The optimal concentration of Ca required is 3mM. An antagonism between Ca and Mg has been demonstrated and it was found that an increase in the concentration of one element
increased the requirement for the other.
Microelements
The microelements viz., Fe, Mn, B, Zn, Mo, Cu, I and Co have a profound effect on growth of tissue in vitro. The availability of the iron is reduced at high pH due to precipitation. To avoid this, Fe is supplied as chelated EDTA complex. These elements produce toxic effect, if they are applied at higher level. A good growth of tissue can be achieved when the concentration of microelements was reduced to 10 per cent of the original level.
Organic nutrients
Carbohydrates
Carbohydrates are used as carbon sources. The standard carbon source is sucrose, at a concentration of 2-5 per cent. Monosaccharides like glucose or fructose can also be used as carbon sources but are generally less suitable. Sucrose is the best source, since Sucrose is dehydrolyzed into usable sugars during autoclaving.
Vitamins
Vitamins are supplemented with medium to achieve the best growth of the tissues. Among the vitamins only thiamine HCL (B1) seems to be universally required. Other vitamins are pyridoxine HCL (B6), nicotinic acid (B3) and calcium pantothanate (B5). Specific requirement of each one varies with the plant species subject to culture.
Phytohormones
These are organic compounds, other than nutrients, which influence growth, differentiation and multiplication. They required in very minute quantity in the media. The requirement for these substances varies considerably with the tissue and it also depends on their endogenous level. There are many commercially available synthetic substances that mimics the PGR specific to certain species. Testing of various types, concentrations and mixtures of growth substances during the development of a tissue culture protocol for a new species is essential before using a new PGR in plant tissue culture. There are different groups of PGRs commonly used in the media. They are auxins, cytokinins, gibberellins, ethylene and absicissic acid. Additional substances gaining recognition as hormones in plant tissue culture are: polyamines, jasmonates, salicylic acid and brassinosteroids.
- Auxin
In nature, the hormones of this group are involved with elongation of stem, internodes, tropism, apical dominance, abscission, rooting etc. In tissue culture auxins have been used for cell division and root differentiation. The commonly used auxins in tissue culture are Indole Acetic Acid(IAA) Auxins are usually dissolved in either ethanol or dilute NaOH.
- Cytokinins
These hormones are essential for cell division, modification of apical dominance, shoot differentiation etc. In tissue culture media, cytokinins are incorporated mainly for cell division, differentiation of adventitious shoots from callus and organ & shoot proliferation. Commonly used cytokinin are eg .Benzyl amino purine (BAP),Isopentenyl adenine (2-ip),Furfurylamino purine (kinetin),Zeatin. Cytokinin are generally dissolved in dilute HCl or NaOH. Auxin – Cytokinin Interaction High auxin and low cytokinin ratio: Initialize root formation, embryogenesis and callus formation. Low auxin and high cytokinin ratio: Induce formation of adventitive or axillary shoots.The auxin-cytokinin ratio is also essential for chloroplast formation and other processes.
- Gibberellins
Naturally occurring plant hormones involved in internode elongation, enhancement of flower, fruit and leaf size, germination and vernalization in plants. Among the 20 known gibberellins, GA3 is used widely. Compared to auxins and cytokinin, gibberellins are used very rarely. they stimulate normal development of plantlets from in vitro formed adventitious embryos.
They are soluble in cold water. - Ethylene
A gaseous plant hormone involved in fruit maturation, abscission, and senescence. All kinds of plant tissue cultures produce ethylene and the rate of production increases under stress conditions. Use of ethylene precursor (2-chloroethylphosphonic acid) in tissue culture may be promotory or inhibitory for the same process in different species. For example, it
promoted somatic embryogenesis in Zea mays whereas the same process was inhibited in Hevea brasiliensis. - Abscisic acid
A plant hormone involved in abscission, enforcing dormancy and regulating early stages of embryo development. It is required for normal growth and development of somatic embryos and promotes morphogenesis. - Brassinosteroids
It promotes shoot elongation at low concentrations and strongly inhibits root growth and development. It also promotes ethylene biosynthesis and epinasty. - Jasmonates
Jasmonates are represented by jasmonic acid and it is a methyl ester. Jasmonic acid is considered to be a new class of plant growth substance. It inhibits many processes such as embryogenesis, seed germination, pollen germination, flower bud formation, chlorophyll formation. It is involved in differentiation, adventitious root formation, breaking of seed
dormancy and pollen germination. - Polyamines
There is some controversy as to whether these compounds should be classified with hormones. They appear to be essential in growth and cell division.
Types of tissue culture
- Seed Culture.
- Embryo Culture.
- Callus Culture.
- Organ Culture.
- Protoplast Culture.
- Anther Culture.
Stages in micropropagation
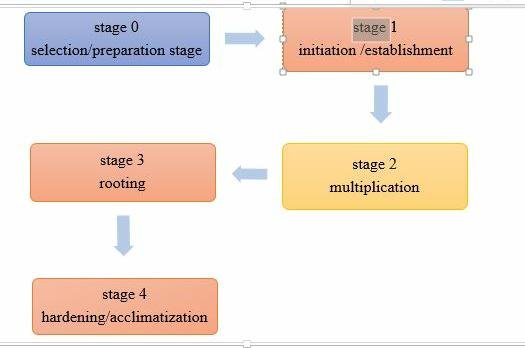
Micropropagation generally involves five stages. Each stage has its own requirements.
Stage 0: Preparative stage
this stage involves the preparation of mother plants to provide quality explants for better establishment of aseptic cultures in stage to reduce the contamination problem in the subsequent stages, mother plant should be grown in a glasshouse and watered so as to avoid overhead irrigation. This will also reduce the need for a harsh sterilization treatment. Stage 0 also includes exposing the stock plants to suitable light, temperature and growth regulator treatments to improve the quality of explants. In the case of photosensitive plants it may be possible to obtain suitable explants throughout the year by controlling photoperiod in the glasshouse. For example, red-light treated plants of Petunia provided leaf explants which produced up to three times as many shoots as did the explants from untreated plants.
Stage 1. Initiation of culture
- Explant: The nature of explant to be used for in vitro propagation is governed by the method of shoot multiplication. For enhanced axillary branching, only the explants which carry a pre-formed vegetative bud are suitable. When the objective is to produce virus-free plants from an infected individual it becomes necessary to start with sub-millimeter shoot
tips. If the stock is virus-tested or virus eradication is not necessary, then the most suitable explant is nodal cuttings. Small shoot-tip explants have a low survival rate and show slow initial growth. Meristem tip culture may also result in the loss of certain horticultural characteristics which are controlled by the presence of virus, such as the clear-vein character of the Geranium cv. Crocodile. Generally, the clear vein character is transmitted in petiole-segment culture but not in shoot-tip culture. - Sterilization: Special precautions need to be taken when explants are derived from field- grown materials, which is often necessary in cloning an elite tree. In such cases an ideal approach would be to take cuttings from the selected plant and grow them in greenhouse. Discarding the surface tissues from plant materials while preparing the explants also
minimizes the loss of cultures due to microbial contamination. - Browning of medium: A serious problem with the culture of some plant species is the oxidation of phenolic compounds leached out from the cut surface of the explant. It turns the medium dark brown and is often toxic to the tissues. This problem is common with the adult tissues from woody species. Stage 2. Multiplication
This is the most crucial stage since it is the point at which most of failures in micropropagation occur. Broadly three approaches have been followed to achieve in vitro multiplication. - Stage 3 Rooting rooting of shoot Somatic embryos carry a pre-formed radical and may develop directly into plantlet However, these embryos often show very poor conversion into plantlets, especially under invitro conditions. They require an additional step of maturation to acquire the capability for normal germination. Adventitious and axillary shoots developed in cultures in the presence of a cytokinin generally lack roots. To obtain full plants the shoots must be transferred to a rooting medium which is different from the shoot multiplication medium, particularly in its hormonal and salt compositions. For rooting, individual shoots measuring 2 cm in length are excised and transferred to the rooting medium.
- Stage 4. Transplantation
The ultimate success of commercial propagation depends on the ability to transfer plants out of culture on a large scale, at low cost and with high survival rates. The plants multiplied in vitro are exposed to a unique set of growth conditions (high levels of inorganic and organic nutrients, growth regulators, sucrose as carbon source, high humidity, low light, poor gaseous exchange) which may support rapid growth and multiplication but also induce structural and physiological abnormalities in the plants, rendering them unfit for survival under in vivo conditions. The two main deficiencies of in vitro grown plants are – poor control of water loss and heterotrophic mode of nutrition. Therefore, gradual acclimatization is necessary for these plants to survive transition from culture to the greenhouse or field. During acclimatization the in vitro formed leaves do not recover but the plant develops normal leaves and functional roots. while transferring out shoots/roots their lower part is gently washed to remove the medium sticking to them. The individual shoots or plantlets are then transferred to potting mix and irrigated with low concentration of inorganic nutrients. This probably recommissions the photosynthetic machinery of plants, enabling them to withstand the subsequent reduction in the ambient relative humidity and survive under field conditions. A variety of potting mixtures such as peat, perlite, polystyrene beads, vermiculate, fine bark, coarse sand etc. or their mixtures in different combinations are used for transplantation. For initial 10-15 days, it is essential to maintain high humidity (90-100%) around the plants, to which they got adapted during culture.
Sterilization
The explant is the tissue or plant part you introduce in the culture media for the regeneration of the plant. Therefore, it’s primary to sterilize it well to remove all the dirt, bacteria, or infections stuck on the surface of the explant. It ensures that the resulting plant will be free from any diseases. The success of tissue culture is based on three main factors: To develop contamination-free cultures.Induction of plantlet in vitro.Transfer of in vitro regenerated plantlets to field conditions. thus, it’s necessary to carefully perform each step for higher yield! So far many surface sterilization techniques have been developed to clean explants. And, this study covers some of them. Further, it also presents a general procedure to sterilize explants and tells how you can use PPM for the purpose.
TYPES OF EXPLANT STERILIZATION TECHNIQUES
Here’re some chemicals most frequently used in labs to sterilize plant explants:
Sodium hypochlorite: It is also known as bleach. It’s one of the most commonly used explant sterilizing chemicals. To use bleach in tissue culture, it is diluted to 10-20%, which results in 0.5-1.0% bleach in the final solution. Each type of explant requires a different amount of time to sterilize. Most of the time, however, 10-20 minutes will suffice.Mercuric chloride: The chemical is highly toxic and that’s why it’s rarely used in labs. When sterilizing the explant using this chemical, it’s essential to rinse it many times using sterile distilled water. There shouldn’t be any traces of compound on the explant. Also, follow all precautions and take extensive care while working with the chemical.
Hydrogen peroxide is a hazardous chemical that is rarely used in the sterilization process. Therefore, all precautions must be taken to sterilize the explant. The concentration most commonly used in the process is around 30%.Calcium hypochlorite: Mix the chemical with water and run it through a filter before using it to sterilize something. The concentration of the explant commonly used to clean the explant is 3.25%.Ethanol: Ethyl alcohol or ethanol is one of the extensively used chemicals for explant sterilization. However, only a few seconds of exposure must be given to the explant because it’s highly phytotoxic. Longer exposure time will lead to tissue damage.PPM (Plant Preservative Mixture): PPM is a robust broad-spectrum formulation for eliminating all types of contamination from plant tissue culture. Follow the given protocol for surface sterilization of explants: Use 5% v/v PPM solution + 3x MS basal ( inorganic ) salts to surface sterilize explants. Then gently stir/shake the water-rinsed explants for 4-12 hours and without rinsing place the explants onto the proper medium with 0.1% v/v PPM. For seeds, Stir seeds in 4% PPM solution (4ml/96ml water) for 12 hours, after that place in a germination medium containing 0.1%v/v PPM without rinsing.
GENERAL PROCEDURE TO STERILIZE EXPLANTS
The procedure of explant sterilization differs based on the type of explants being used for the tissue culture process. The duration of chemical exposure to the explant and the chemical used is also decided based on the nature of the explant. Because too much exposure to harsh chemicals to soft explants can damage their tissues. Here’s a general explant sterilizing method to obtain infection-free explants for your tissue culture processes:Using tap water remove dust or any other external material attached to the plant part or tissue.Wash it again using distilled water.Now take some amount of distilled water in a beaker and add a few drops of detergent, such as Tween 20. Put the explant in the solution with continuously stirring.Rinse the explant twice using sterilized distilled water.Use a sharp scalpel to trim the size of the explant under the laminar hood.Surface disinfect the explants using 70% ethanol for 30 seconds. (Some procedures use HgCl2 (mercury chloride), NaOCl (sodium hypochlorite), and bromine water to disinfect explants).
Tools using in Plant Tissue culture
Autoclave
Autoclave is used to sterilize medium, glassware and tools for the purpose of plant tissue culture. The same equipment is used in hospitals to sterilize gauge, cotton, tools and linen, etc. Sterilization of material is carried out by increasing moist heat (121 °C) due to increased pressure inside the vessel (15-22 psi, pounds per square inch or 1.02 to 1.5 kg/cm2) for 15 minutes for routine sterilization. Moist heat kills the microorganism and makes the material free from microbes.
Construction :
Autoclaves of different sizes from 5 litres to several hundred litres capacity are available in horizontal or vertical designs.an autoclave have a body, an internal (or external for small sized autoclaves comparable to household pressure cooker) heating system, a container to hold material, its cover fixed with pressure gauge, safety valve, pressure release valve etc. Lid is tightened with the help of screws and a gasket seals the body and lid. A jacket, paddle lifter, timer, and indicator etc., are also provided with large sized autoclaves
Autoclaves may be constructed of aluminum, mild steel, stainless steel or gun metal. Industrial autoclave can accommodate large trolley containing huge number of glassware’s or large bioreactors.
Operation:
Place the materials (wrapped in aluminum foil or paper or in metal box) and glass-wares containing medium (plugged with non-absorbent cotton and covered with aluminum foil) in the bucket. Check water level for appropriate level, tighten all the screws, and switch-on the current. Allow the steam to pass freely from release valve for 5 minutes and then close the valve After attaining a pressure of 15 psi, count 15 minutes for sterilization and then switch-off the current. Pressure is maintained by safety valve. Modern autoclaves are fitted with temperature and time control units and can automatically control the period for sterilization and then switch-off themselves. Empty vessels, beakers, graduated cylinders, etc., should be closed with a cap or aluminum foil. Tools should also be wrapped in foil or paper or put in a covered sterilization tray. For sterilization to work, it is important that the steam gets inside the items. For large sized vessels and large volume flasks containing high amounts of liquid, the duration for sterilization should be increased accordingly. shows the relationship between the volume of the solution and its sterilization time at 15 psi. For proper sterilization, the vessels should not be filled more than one-third of their capacity.
Precautions:
- Check water level each time, the heating elements should remain immersed in the water.
- Check spring of safety valve frequently and clean opening whenever necessary.
- Opposite screws of the lid should be tightened simultaneously.
- Do not over tighten the screws to avoid damage to the gasket.
- Use permanent marker to mark your flasks and its medium.
- All the electrical equipment should be properly earthen to avoid electric shock.
Laminar Air flow

Laminar air flow (LAF) bench is the main working table for aseptic manipulations related to plant tissue culture. This is equipment fitted with High Efficiency Particulate Air (HEPA) Filters, which allow air to pass but retain all the particles and micro-organisms. These HEPA Filters have a very small pore size (0.3 µm) with 99.97-99.99% efficiency UV is switched-on for 30 minutes before starting the work to make area free from microbes. LAF Bench of steel and wooden cabinets is available with different working table size and for vertical (downward air flow) or horizontal (horizontal flow) model. There are several manufacturers in India for this instrument (body) but HEPA filters are imported HEPA filters are also used to create ‘clean area’ for culture rooms and inoculation room etc. If LAF bench is placed in such a clear area, efficiency and life of the equipment are increased. air blown by a blower is passed through these filters, thus, always generally a positive pressure of air is maintained from inside to outside. This positive pressure does not allow any particle to enter in the working area of LAF bench. A manometer measures the air pressure inside the instrument; pressures greater than 12 bars indicate filter clogging, and filters should be replaced at this point. Equipment is fitted with UV light and visible light source UV is switched-on for 30 minutes before starting the work to make area free from microbes. LAF Bench of steel and wooden cabinets is available with different working table size and for vertical (downward air flow) or horizontal (horizontal flow) model. There are several manufacturers in India for this instrument (body) but HEPA filters are imported HEPA filters are also used to create ‘clean area’ for culture rooms and inoculation room etc. If LAF bench is placed in a clear area.
Work Done
Day 1 Assignment 1 Date 19/12/2022
MS media Preparation
Lab cleaning, lab rearrangement. 1st day of training we learnt about lab cleaning.
- lab were cleaned by using Dettol and Ethanol
- Rearrange all the instruments which is needed for the tissue culture.
- All glassware Bottles, tubes and Petri plates were cleaned and dried using Hot Air Oven and Autoclaved all of them.
2. Distilled water collection and sterilization.
Stored the Distilled water and sterile the water in autoclave for the media preparation
Day 2 20/12/2022
preparation if MS Media for inaculation of tobacco seed and turmeric plant .Make a stock solution first, then prepare the final volume of MS Media.MS media containing macronutrients and micronutrients.vitamins plant growth hormone
DAY 3 20/12/2022
collection of mother plant
Tobacco seed and Turmeric
Inoculation and labelling
- Tap water washing with fungicide Bavistin for 30 min.
- Washing with distilled water inside laminar.
- Sterile distilled water, glasswares,culture water and miscellaneous and UV treatment 20-30 min.
- Wash explant with distilled water twice.
- Deep explant in 0.1% HgCl2 and wait for 2-3 min
- 3-5 times wash with D/W
- Deep explant in 70% alcohol leaf or nodule section wait 30 sec-1 min
- 3-5 times wash with D/W
- Drain out sterilized explant for inoculation.
- cut down small bud of turmeric by using sterile surgical blade and then cut leaf into 4 parts.
- by using sterile forceps place the turmeric explant in media bottle.
- place the explant at middle of bottle and make sure that don’t place explant very close to edge of bottle.

Date 05/01/20223
sub culturing of tobacco leaf.
19/02/2023
patade sir visiting our lab some suggestion they told us
All lab cleaning
packing of holes
positive pressure
pass box
glass beads sterilizers
Date 6/03/2023
subculturing of tobbaco plant
MS media preparation
- stock 1 – Macronutrient (major stock )
| Sr. no. | Chemical | Weight ( mg/lit) | Weight for 20X (mg) for 500ml |
| 1 | NH4NO3 | 1650 | 33000 |
| 2 | KNO3 | 1900 | 38000 |
| 3 | MgSO4.7H2O | 370 | 7400 |
| 4 | KH2PO4 | 170 | 3400 |
| 5 | CaCl2.2H2O | 440 | 8800 |
Macronutrient stock
Dissolve all chemicals should be dissolve one by one in 500 ml D/W.
NOTE:-To make 1 liter MS basal media we have to add 25 ml of macronutrient stock.
- stock 2 – Micronutrient
| Sr.no. | Chemicals | Weight ( mg/lit) | Weight for 200X (mg) for 500ml |
| 1 | H3BO3 | 6.2 | 1640 |
| 2 | MnSO4.4H2O | 22.3 | 4460 |
| 3 | ZnSO4.4H2O | 8.6 | 1720 |
| 4 | KI | 0.83 | 166 |
| 5 | Na2MoO4 | 0.25 | 50 |
| 6 | CoCl2.6H2O | 0.025 | 5 |
| 7 | CuSO4.5H2O | 0.025 | 5 |
Micronutrient stock
NOTE:-To make 1 liter MS basal media we have to add 2.5 ml of micronutrient stock.
- Stock3 -Fe-Na- EDTA
| Sr.no. | Chemical | Weight ( mg/lit) | Weight for 50X (mg) for (200ml) |
| 1 | Na2EDTA.2H2O | 37.3 | 1865 |
| 2 | FeSO4.7H2O | 27.8 | 1390 |
Fe-Na- EDTA stock
NOTE:-To make 1 liter MS basal media we have to add 1ml of Fe-Na- EDTA stock .
- Stock-4 Vitamin
| sr.no. | Chemicals | mg/lit. | 100x mg |
| 1 | Thiamin HCl | 0.1 | 10 mg |
| 2 | Nicotinic acid | 0.5 | 50 mg |
| 3 | Pyridoxin HCl | 0.5 | 50 mg |
| 4 | Glycine | 2.0 | 200 mg |
- Myo-inositol – 100mg/Lit to be added freshly
- Sucrose 30g/ lit
- pH= 5.8
- agar agar -8 gm/lit
Note- for pH calibration Chemical should be used
| 0.1 N NaOH | 100 ml |
| 0.1 N HCl | 100 ml |
- PGR stock – Auxin and cytokine 100, 50 mg/ml
- MS basal media for seed germination (1000 ml)
- MS+ 0.55 mg/liter BAP (500 ml)
- MS + 1 mg/liter BAP ( 500 ml)
Sterilization of Glassware: –
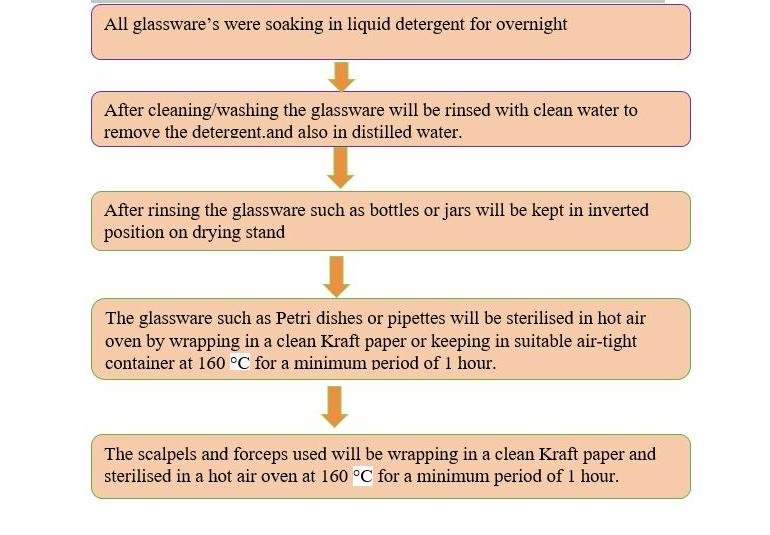
The glassware such as culture bottles, measuring cylinder and the other equipment like forceps, cutting paper and blade holder were washing using detergent. Before being dried on oven they were rinsed in distilled water, wrapped in aluminum foil and subsequently autoclaved at 121oC at 15 psi pressure for 20 min.
Inoculation Procedure:
- Laminar air flow was switched on and working area were sterilized.
- The sterilized equipments, glassware’s, culture bottles and distilled water were then kept
on the working area of Laminar Air Flow. - Surface sterilized explants were washed with doubled distilled autoclaved water for 2-3
times under Laminar Air Flow sterile condition. - Forceps and cutting blades were kept in glass bead sterilizer for surface sterilization.
- Previously sterilized Petri plate was placed on the working area of Laminar Air Flow,
then it was over layed with autoclaved filter paper. - One by one explants were placed on the filter paper to soak up the extra water. The nodal
explants were then cut from both ends. - Finally, with the help of forceps the explants were inoculated on the surface of the MS
media in a such a way that ¾ part of the nodal explants would be in contact with MS
media. - The culture bottles containing explants inoculated on MS media supplemented with
respective different conc. of different concentration of cytokinin BAP (0.5-4.0mg/L)
combination with IAA (0.5mg/L) were incubated at 25±5°C under white fluorescent light
(2000 lux) for 16/8 hours light and dark conditions. - For Shoot initiation, the excised explants were transferred on MS medium supplemented
with different concentrations of hormones (BAP & IAA).
The initiation medium was 5 types: - MS medium with 0.5 (mg/L) BAP + 0.5 (mg/L) IAA
- MS medium with 1.0 (mg/L) BAP + 0.5 (mg/L) IAA

Culture Maintenance & Conditions:
All cultures were maintained at 25±2̊C and 60-70% Relative Humidity in a culture room
Under the warm white fluorescent light intensity varied from 2000-3000 lux with a photoperiod
of 18 hrs. day light and 6 hrs. dark.

Results
contaminated bottles were observed in culture room

shoot culture of Frerea indica ( Shiv suman )

APRIL 2023
Training of plant tissue culture in Junnar College
Date 9 April 2023 to 12 April
- Cleaning plant tissue culture lab.
- Fumigating lab.
- Test tube cleaning soaking in liquid soap washing distilled water rinsing and drying.
- Distilled water collection.
- Chemicals arrangement.
- Attending the lecture on MS media preparation
Date 19 to 20 April
- Making cotton plug
- Autoclaving culture bottles other glassware, and distilled water.
Date 27 April to 29 April
Preparation of MS stock solution
- Major stock I 500 ml
- Minor stock II 100 ml
- Iron stock III 100 ml
- Vitamin stock IV 100 ml
- Preparing full Ms Media without sucrose 500ml
- Preparing half MS media without sucrose 500ml
- Preparing Ms basal media without PGR 250 m

cotton plug making
MONTH MAY
Lab Work
- Soaking glassware in liquid soap, washing glassware, drying in a hot air oven, and keeping it in a culture room.
Training of plant tissue culture in Junnar College
Date: 2 may 2023
- Cleaning PTC lab.
- Making MS media 1500 ml
- Preparations of plant growth regulators (PGR).
- Autoclaved all media and poured in test tubes, packed with cotton plug.
Date: 3 may 2023
- Test tubes were autoclaved.
- Distilled water collection
- Washing culture bottles and dry.
LAB SETUP
This month we learned how to make plant tissue culture media .
sterilization process
Sterilization of culture room
Floor and walls are washed first with detergent and then with 2% sodium hypochlorite or 95% ethanol. The cabinet of laminar airflow is sterilized by clearing the work surface with 95% ethanol and then exposure of UV radiation for 15 minutes
Sterilization of Nutrient Media: Culturemedia are dispensed in glass containers, plugged with non-absorbent cotton or sealed with plastic closures and then sterilized using autoclave at 15 psi (121°C) for 15 to 30 minutes.

STERILIZATION OF NAPIER GRASS EXPLANT
Sterilization of Explants: The plant materials to be used for tissue culture should be surface sterilized by first exposing the material in running tap water and then treating it in surface sterilization agents like 0.1% mercuric chloride, 70% ethanol under aseptic condition inside the Laminar Air Flow Chamber.
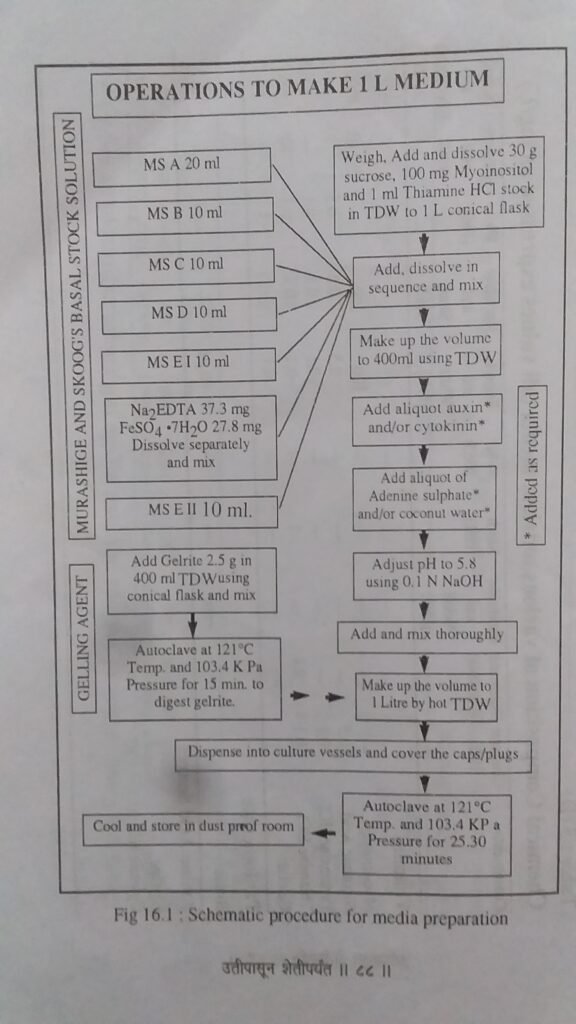
FLOWCHART FOR MEDIA PREPARATION
STERILIZATION STEPS FOR GLASWARES
PLANT TISSUE CULTURE STAGES
LAB DESIGN

after setting up lab we cleaned and fumigated entire lab .then prepared all stock solution freshly .all chemicals arranged in rack then After we prepared the media. The prepared media was firstly autoclaved. Then poured into sterilized culture bottle. and kept in laminar airflow.

stock solution preparation


INOCULATION NAPIER GRASS EXPLANT
then after 2 week we observed the growth of Napier explant formation of small shoot and some test tubes were contaminated

shoot formation in Napier plant
1.firstly collect the Napier explant from Vigyan. Ashram agriculture field .
2. Washed under running under tap water
3.Washed with Bavistin powder for 10 to 15 min
4.then washed again distilled water for. 3 times
then other procedure done in under aseptic condition in laminar air flow
5.laminar air flow cleaned with 70% acetone. And switch ON U.V light for 10 min.
then after 10 min U.V button switch off.and cleaned our hand by Using sanitizer .
and explant of Napier was dipped into 0.1% mercuric chloride. For 1 min.
then washed with d.w for 3 times
then inoculated in to test tube .with help of forceps
then closed with tight cotton plug.
and kept in the culture room.

SEPTEMBER
This month I started working on moringa tree because one of the farmer in pabal village wants plant tissue cultured moringa trees so Ranjeet sir told me about that. So my main focus was on micropropagation of moringa trees in tissue culture lab the nodal segments of drumstick was took as an explant.
This month Avanti madam joined as consultant for plant tissue culture lab they suggest some changes in our lab . I required some glassware and chemicals for further work
MORINGA (DRUMSTICK) INTRODUCTION
NAME : Moringa
common Name : drumstick / shevaga
scientific Name : moringa oleifera
family ; moringaceae
Moringa oleifera (MO) is an indigenous tree from the north of India, Pakistan, and Nepal, of which all its components (leaves, seeds, flowers, and bark) are considered medicinal. Its medicinal components have been used by traditional Ayurvedic medicine.
SUGGESTIONS
Sodium Hypo chloride
test tubes
distilled water assembly
Glassbead sterilizer
glassrod
this are some equipment’s are required for PTC lab

drumstick explant is collected from vigyan ashram campus. Local variety of drumstick plant are available in ashram campus The explant cut in 1 to 2 cm size for the inoculation first the explant washed under running tap water, for removing dust and other contamination.

EXPLANT CUTTING
sterilization of Explant
- washed explant treated with 1% Bavistin for 10 min then washed with 3 times distilled water then
- treated with 0.1% Hgcl for 2 min and again 3 time distilled water washing
- then give 4% sodium Sodium hypochlorite 1 min and washed with 3 time distill water
- NOTE :Hgcl and sodium hypo chloride treatment give in LAF

BAVISTIN TREATMENT
NOTE : 1gm Bavistin dissolved in 100 ml distilled water to make 1% Bavistin Solution

Avanti madam visit
PREPARATION OF PLANT GROWTH REGULATORS
The hormonal combination of BAP (0.5ml/lit) + IAA (0.5ml/lit) were Used as basic media for micropropagation of drumstick
There are mainly two type of hormone we were used
Auxin is a root hormone e.g. IAA,NAA,
cytokinin is a shoot hormone e.g. BAP,Kineatin
NOTE : when preparation of PGR stock that time First auxin and cytokinin dissolved in 0.01 ml NaOH OR ETHANOL then add distilled water

Drumstick Inoculation
OCTOBER
while working in plant tissue culture lab I Observed some problems
contamination causes
- Positive Pressure module
- fogging machine
- isolation Full Apron
- Separate Autoclave machine for discarding
in this month Avanti Madam visited the PTC lab this month on 5 Oct 2023 we made a plant growth regulator (PGR) stock solution.
Made a total of 1.5L MS media with 0.5 mg IAA +1 mg BAP conc.In this month Inoculated 70 test tubes were, the explant is the moringa nodal segment.
| Sr no | Date | Work done |
| 1 | 4 /10/2023 | Cleaning lab |
| 2 | 5 /10/2023 | Avanti madam visit |
| 3 | 6 /10/2023 | Preparation of PGR stock solution |
| 4 | 8 /10/2023 | Soil microbiology workshop conduct |
| 5 | 10/10 /2023 to 14/10/2023 | Makethon |
| 6 | 19/10/2023 | Cleaning lab and shifting microbiology instruments |
| 7 | 21/10/2023 | Autoclaved all contaminated test tubes and bottles and fumigation |
| 8 | 25/10/2023 | Preparation of 4 stock solutions |
| 9 | 26/10/2023 to 28/10/2023 | 3L MS Media preparation autoclaving glassware |
| 10 | 3/11/2023 | Inoculate 30 explants of moringa |
November
Plant tissue culture lab
This month the main focus was on the reduced contamination in the plant tissue culture lab and inoculation of the moringa nodal segment after Bavistin and copper oxychloride treatment this treatment reduced maximum outside contamination of the explant.
Awanti Madam visited in plant tissue culture lab on 6 November and 24 November
The hormonal combinations like B A P (0.5ml/lit) + IAA (0.3ml/lit) were shown growth of shoot after 15 Days of inoculation.

this was first time the formation of shoot by using BAP hormone and IAA hormone.
collection of plant sample

| Sr no | Date | Work done |
| 1 | 4 /11/2023 | Cleaning lab |
| 2 | 6/11/2023 | Avanti madam visit ,collection of explant ,sterilization of explant and inoculation 24 test tube |
| 3 | 7/11/2023 | Preparation of MS media 500 ml |
| 4 | 8 /112023 | Autoclaving media and kept in incubator for 2 days. |
| 5 | 10/11/2023 | Inoculation of explant 24 test tube |
| 6 | 20/11/2023 | Cleaning lab , fumigation of lab |
| 7 | 21/11 2023 | Glassware cleaning and kept in hot air oven |
| 8 | 23/11/2023 | Media preparation 500ml Glassware cleaning sterilization |
| 9 | 24/11/2023 | Avanti madam visit Explant collection, sterilization of explant, and inoculation of 24 test tube |
| 10 | 25/11/2023 | Spraying bavistin on moringa plant Lab cleaning |
| 11 | 27/11/2023 | Distil water collection setup |
| 12 | 28/11/2023 | Lab cleaning ,laminar airflow cleaning and glassware are autoclaved |
| 13 | 29/11/2023 | MS media preparation 500 ml and autoclaving media and kept in incubator |
| 14 | 30/11/2023 | Lab cleaning and glassware cleaning |
| Sr no | Date | Work |
| 1 | 1/12/2023 | Lab cleaning |
| 2 | 2/12/2023 | 2,4, D and NAA hormone purchased from Pune |
| 3 | 4/12//2023 | cleaning lab, inoculation of moringa explant |
| 4 | 5/12/2023 | installation of glassware distilled water unit |
| 5 | 6/12/2023 | making 2,4, D, And NAA hormone stock for subculturing of moringa plant |
| 6 | 7/12/2023 | Avanti Madam visited the plant tissue culture lab we prepared MS media500 ml, poured the media into a test tube, autoclaved media then kept it for incubation |
| 7 | 8/12/2023 | Preparation of NAA and 2,4, D plant growth regulator stock |
| 8 | 11/12/2023 | Discarding contaminated test tube |
| 9 | 14/12/2023 | Soil testing |
| 10 | 15/12/2023 | Purchase order for positive pressure |
| 11 | 16/12/2023 | Soil lime analysis |
| 12 | 18/12/2023 | Inoculation of moringa explant |
| 13 | 19/12/2023 | Avanti Madam visit PTC lab AC EXHAUST fan repairing |
| 14 | 21/12/2023 | Cleaning lab and sterilizing glassware’s |
| 15 | 23/12/2023 | Soil microbiology workshop |
| 25/12/2023 | Chlorophyll estimation trial | |
| 16 | 26/12/2023 | Positive pressure installation |
| 17 | 27/12/2023 | Cleaning lab |
| 18 | 28/12/2023 | Glassware cleaning and sterilization |
| 19 | 29/12/2023 | Inoculation moringa explant 24 test tube |

