INTRODUCTION / NEED OF PROJECT
What is Greywater?
Greywater is defined as wastewater generated from wash hand basins, showers and baths, which can be recycled on-site for uses such as flushing in toilets, landscape irrigation.
- In India, statistics says that around 30 to 50 percent of the wastewater discharged to the sewer is contributed by grey water. Therefore, by recycling it, we can significantly reduce the load on the infrastructure.
- There are many rivers in India like Ganga, Yamuna, Damodar river etc which are getting polluted day by day.
- According to data released by Pune Municipal Corporation’s (PMC) environment department, constantly rising pollution levels since 2012 have now transformed Mutha into a ‘dead’ river. As Increasing urbanization coupled with industrialization during the past few decades are depleting aquatic ecosystems.
- With the rapid increase in the population of the city and the need to meet the increasing demands of human and industrial consumption, the available water resources of the city are getting depleted and the water quality has deteriorated.
OBJECTIVES
- Installation of the system to site at Kanherser.
- To reduce the COD(chemical oxygen demand) of grey water.
- To observe and analyze the data.
METHODOLOGY
- Aeration can be increased by using Airoxi tube.
- Check the reduction of COD by changing bubbling pattern.
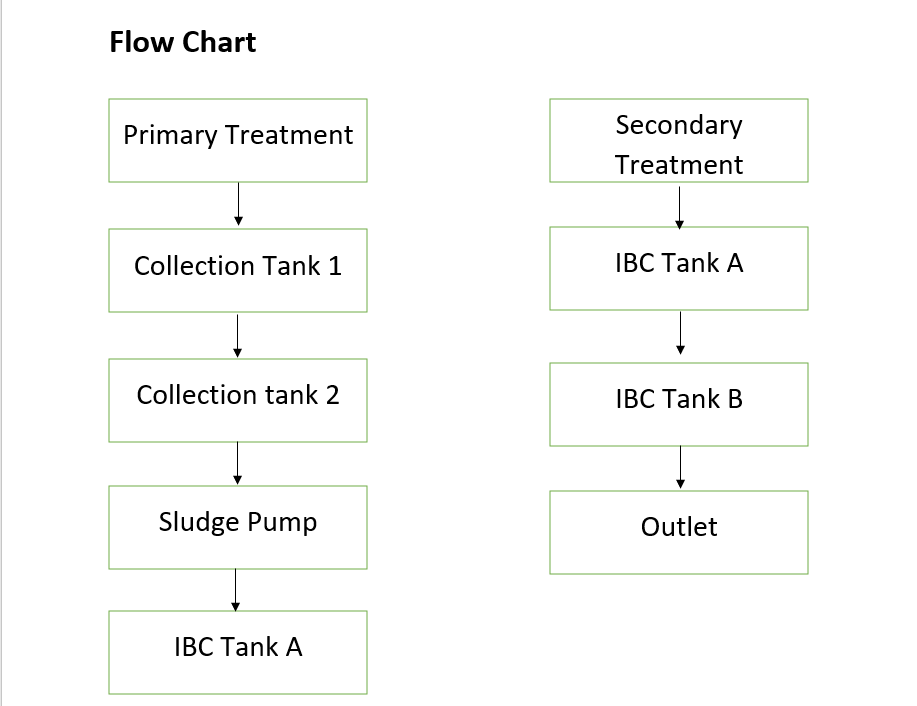
INSTALLATION
10/09/2022
Today we transferred the 2 IBC tanks and solar panels to site at Kanserser.


17/09/2022
Done the fitting of frame of Solar Panel.

20/09/2022
Fixed the solar panels and VFD.

22/09/2022
Put the sludge pump in the primary concrete tank 2. And initially we put the bubbler in the IBC-A & IBC-B.
PROCEDURE FOR DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD)
Materials for COD test
1)Potassium dichromate (K2Cr2O7).
2) Silver sulphate (Ag2SO4)
3)Sulfuric acid (H2SO4)
4)Ferrous ammonium sulphate (FAS)
5)Ferron indicator
6)Potassium hydrogen phthalate (KHP)
7)250 ml Erlenmeyer flask
8)Reflux condenser
9)COD tubes
10)Measuring cylinder
11)Analytical balance
12)COD tube stands
13)Hand gloves
Preparation of chemicals
1)Preparation of 0.25 N potassium dichromate solution: –
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask.
2) Preparation of Ammonium Ferrous Sulphate solution: –
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid.
3) Preparation of Potassium Hydrogen Phthalate solution: –
0.425 gm. of dried KHP powder dissolved in 1000 ml distilled water in 1000 ml
volumetric flask. (Dried this powder for 2 hours in oven)
Procedure: –
1)Took 0.4 gm. Ag2So4 (silver sulphate) in COD tubes.
2) Added 20 ml sample in each COD tube
3)Added 20 ml distilled water in each COD tube.
4) Added stones (crush on beads) 1 or 2 pieces.
5) Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
6) Added 30 ml conc sulfuric acid slowly along with swirling.
7) If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
8) Kept COD tubes in COD apparatus to digest the sample. Attached the condenser & set 150 Degree Celsius temperature.
9) Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
10) After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
11) Took the above sample in a conical flask.
12)Added 150 ml distilled water.
13) Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
A-Reading blank solution.
B-Reading with sample solutions
N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
23/09/2022
Today morning we transferred the grey water from Collection tank 2 to the IBC tanks.

Took the sample at 10 am and 4pm after 6hrs bubbling from IBC Tank B. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10 | – |
| KHP | 5 | 500 |
| Sample 1 | 7.5 | 2500 |
| Sample 2 | 8.5 | 1500 |

24/09/2022
Took the sample at 10 am and 4pm after 6hrs bubbling from IBC Tank B. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10 | – |
| Sample 1 | 8 | 2000 |
| Sample 2 | 9 | 1000 |
26/09/2022
Took the sample at 10 am and 4pm after 6hrs bubbling from IBC Tank B. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 7.5 | – |
| Sample 1 | 6.6 | 900 |
| Sample 2 | 7 | 500 |
10/10/2022
Today we emptied the IBC tanks and took the new water from collection tank 2. And put the airoxi tube to the bubbler pipe.

As time taken to reduce COD was more so we decided to put the bubbling Primary compartment 2 and IBC tank A
Took the sample at 10 am from IBC Tank A and Primary compartment 2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 12 | – |
| Primary Compartment 2 | 9 | 2700 |
| IBC tank A | 10..5 | 1800 |

11/10/2022
Took the sample at 10 am from IBC Tank A and Primary compartment 2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 11.1 | – |
| Primary Tank 2 | 9.5 | 1600 |
| IBC tank A | 10 | 1100 |
12/10/2022
Took the sample at 10 am from IBC Tank A and Primary compartment 2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10.2 | – |
| Primary Tank 2 | 9.1 | 1000 |
| IBC tank A | 9.8 | 400 |
17/10/2022
Today we drilled and fitter the joiner for outlet for IBC tank B

18/10/2022
Took the sample at 10 am from IBC Tank A and Primary compartment 2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10.5 | – |
| KHP | 5.1 | 5100 |
| Primary compartment 2 | 10 | 500 |
| IBC Tank A | 10.2 | 300 |
Observations:
- Smell was reduced.
- Color was changed.
- Retention time not reduced.
As to reduce the retention time we decided to put the 3rd bubbler in Primary compartment 1. Took new bubbler and placed separate bubblers to Primary collection tanks 1&2 and IBC tank A


21/10/2022
Took the sample at 12 pm from IBC Tank B Primary Collection tank 1&2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10.5 | – |
| Primary 1 | 9.8 | 700 |
| Primary 2 | 10.1 | 408 |
| IBC A | 10 | 510 |
28/10/2022
Split the 1st bubbler with IBC tank A and IBC tank B
Took the sample at 11 am from IBC Tank A&B Primary Collection tank 1&2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10.6 | – |
| Primary 1 | 9.8 | 400 |
| Primary 2 | 9.9 | 300 |
| IBC A | 10.2 | 400 |
| IBC B | 10.1 | 300 |
29/10/2022
Took the sample at 11 am from IBC Tank A&B Primary Collection tank 1&2. And found the COD.
| Sample | Reading | COD (mg/lit) |
| Blank | 10.5 | – |
| Primary 1 | 9.9 | 600 |
| Primary 2 | 10.15 | 350 |
| IBC A | 10.2 | 300 |
| IBC B | 10.28 | 220 |
Observations:
- COD of the primary collection tank is reduced.
- Retention time reduced.
- Smell is totally removed .
- Color of the water has become more transparent.
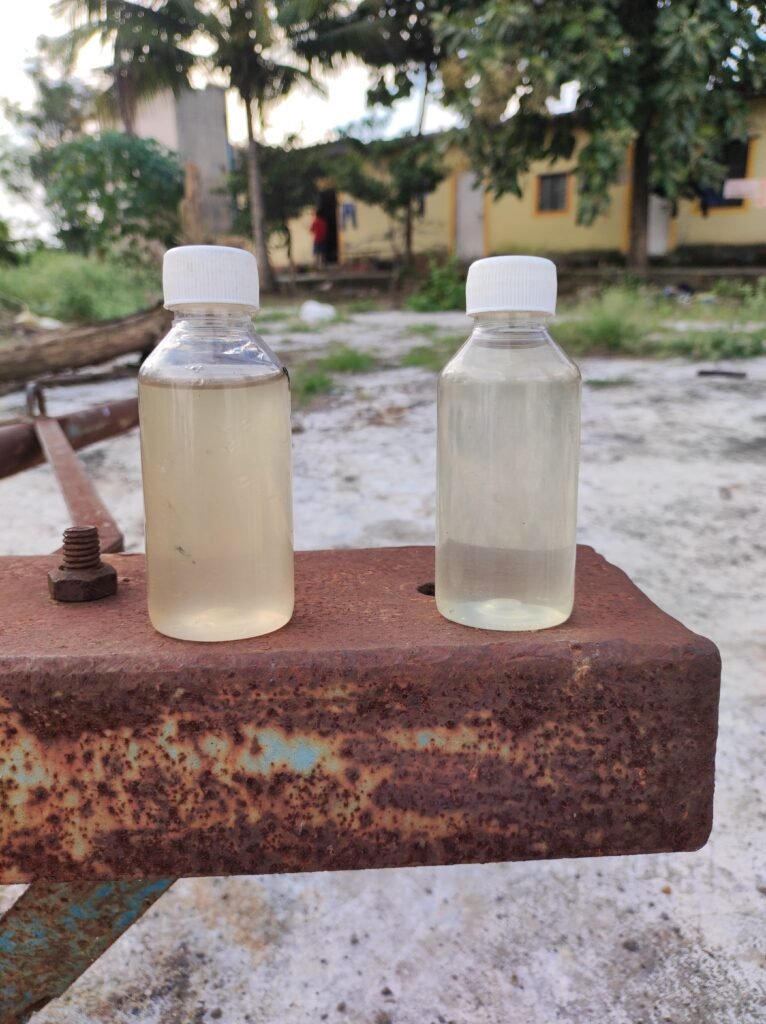

CONCLUSION
- Greywater reuse is an increasingly common household practice in a number of countries. A range of technical solutions are available and there are simple ways to minimize the potential adverse impacts on plant and human health.
- The reuse of greywater will solve many problems related to water scarcity and also water pollution in rivers will be decreased if grey water system are used for treatment waste water.
- The Chemical Oxygen Demand (COD) can be reduced by bubbling in all the compartment.
- With the help of 3 bubblers of 45 Watt we reduced the Chemical Oxygen Demand (COD) from 2500mg/lit to 220mg/lit and the continuous system is set as the bubbling is present in all the compartments.
- Overall this project was useful and also helpful in studying the importance of primary treatment of water and also learned the process of how to find out (COD) of water.




