Joining Date :19|08|2022
I like to learn new things that’s why I came vigyan ashran and whenever I came here I realized that. Yes, I will definetly learn more and more new thing. Actully My real learning day will Start from tommorow I am just eagerly waiting for that day.
My first activity
Soil Testing
20|08|2022
Soil testing elements detection (NPK),PH test,TDS,EC.
Soil testing provides valuable information on pH and plant-available nutrients.
Essential elements of soil testing that are nitrogen, phosphorus, potassium.
All elements in soil that are ..
Apparatus used for soil testing:
Weighing balance, glass beaker, measuring cylinder, various reagents used for soil testing, test tube, funnel, filter paper, tissue paper, cotton, butter paper
soil testing
Test for Nitrogen:
Test for Phosphorus:
Test for Sulfur:
Test for PH:
REVIEW OF PROJECT
After a review of the first assignment and start of the project, Sonal Shinde mam gave us a project on soil analysis in presence of also Dikshit sir and all our Copartners. He also introduced us about soil lab and instruments used for the soil analysis.
Also, they remind us all the assignment and work which we have done in last few days. They asked some questions related to project and the activities which had done in few past days. All these things are Important for future used. Also they told us to update our blog regularly so that after me other can use the things which l have done on my project.
Result and conclusion:
In a soil we observed NPK elements are presents in essential amount and PH . I learned lot of things and I am definately said that I can test different type of soil. Also learned which elements in soil.
___________________________________________________________________________________________________________________________________________
21/08/2022
Holiday : searching on study in computer lab
That are related to blog and all.
___________________________________________________________________________________________________________________________________________
22/08/2022
Lab work
cleaning and knowing all things
Activity : working on Auto clave, Laminar air flow.
1) Autoclave
Aim: To study Autoclave principal, working and uses.
Principal:
The basic principle of steam sterilization, as accomplished in an autoclave, is to expose each item to direct steam contact at the required temperature and pressure for the specified time. Thus, there are four parameters of steam sterilization: steam, pressure, temperature, and time.
Working:
A sufficient quantity of water is poured into the chamber and the level of water is adjusted in such a way that it is slightly touching to the bottom of the perforated chamber. The material is packed in the perforated chamber. The lid is then closed with the screw and the autoclave is switched on. Initially, discharge tap is kept open and safety valve is adjusted to required pressure. As the temperature of water increases, it starts to vaporise and result in the formation of stem. This steam removes the air from autoclave which is indicated by formation of air bubble at discharge valve. When air bubbles stop emitting from discharge tap, it indicates that complete air has been removed out. At this stage the discharge valve is closed. Once steam pressure reaches to desired value, safety valve is set open. From this point subject is hold for time as specified in table 1. After holding time autoclave is switched off and allows cooling. Then lid is opened and sterilized materials are taken out.

Applications of Autoclave:
1. Autoclaves are widely used in microbiology, veterinary science, mycology etc.
2. It is used to sterilize wide range of material including but not limited to laboratory glasswares, laboratory equipments and instruments, surgical material including needles, seizures, heat-stable hand gloves, containers, and closures etc.
3. Autoclave is most commonly involved in the sterilization of biological media
2)Laminar air flow:
Aim: To study laminar air flow principle, working and uses.
Principal:
Laminar airflow, also known as laminar air flow (LAF), is a device designed to prevent the equipment and working environment from particles. Laminar airflow units create particle-free working environments by sucking air through a filtration system and exhausting it across a work surface in a laminar air stream.
Working:
Laminar air flow unit works by the use of in-flow laminar air drawn through one or more HEPA filters, designed to create a particle-free working environment and provide product protection. Air is taken through a filtration system and then exhausted across the work surface as part of the laminar flow process.
Commonly, the filtration system comprises a pre-filter and a HEPA filter.
First, the air goes through the pre-filter (panel filter). Next, the fan blows the air towards the HEPA filter. HEPA filter captures the dust, bacteria, and airborne particles. Therefore, the HEPA filtered air is clean and particle-free.

Application:
Laminar flow cabinets are used in laboratories for contamination sensitive processes like plant tissue culture.
Other laboratories processes like media plate preparation and culture of organisms can be performed inside the cabinet.
Operations of particle sensitive electronic devices are performed inside the cabinet.
In the pharmaceutical industries, drug preparation techniques are also performed inside the cabinet to ensure a particulate-free environment during the operations.
Laminar flow cabinets can be made tailor-made for some specialized works and can also be used for general lab techniques in the microbiological as well as the industrial sectors.
conclusion :
In my perspective its very good way to learned many things and I realized I got step by step knowledge .
__________________________________________________________________________________________________________________________________________
23/08/2022
Activity : distillation of water
Distillation process.

Oven dryer:
Aim:To study Oven dryer principal, working uses
Principal:
Sterilization by dry heat is performed by conduction. The temperature is consumed by the surface of the objects, then moves towards the core of the object, coating by coating
Working:
. Heating Mechanism:- Killing effect of dry heat on microorganisms is due to i) destructive oxidation of essential cell constituents, ii) protein denaturation and iii) toxic effect of elevated levels of electrolytes

Uses:
Sterilization of articles that withstand high temperature and do not get burned e.g. Glass-wares, powders, forceps, scissors, scalpels, glass syringes, pharmaceutical products like liquid paraffin, fats, grease, and dusting powder, etc.
Conclusion :
In this day i learned various basic concept about distilation process in and oven dryer in which how to set? how to used? just like all things.
________________________________________________________________________________________________________________________________________
24/08/2022
Activity :Determination of CODin different type of solutions.
Chemical oxygen demand is amount of dissolved oxygen that must be present in water to oxidize chemical organic materials. The initial COD of black water is needed to determine. Sample for determining COD in different solutions.

Materials for COD test
1)Potassium dichromate (K2Cr2O7).
2)Mercuric sulphate (HgSO4)
3)Sulfuric acid (H2SO4)
4)Ferrous ammonium sulphate (FAS)
5)Ferron indicator
6)Potassium hydrogen phosphate (KHP)
7)250 ml Erlenmeyer flask
8)Reflux condenser
9)COD tubes
10)Measuring cylinder
11)Analytical balance
12)COD tube stands
13)Hand gloves

Preparation of chemicals
1)Preparation of 0.25 N potassium dichromate solution: –
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask.
2) Preparation of Ammonium Ferrous Sulphate solution: –
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid.
3) Preparation of Potassium Hydrogen Phosphate solution: –
0.425 gm. of dried KHP powder dissolved in 1000 ml distilled water in 1000 ml
volumetric flask. (Dried this powder for 2 hours in oven)
Procedure:-
1)Took 0.4 gm. AgSo4 (silver sulphate) in COD tubes.
2) Added 20 ml sample in each COD tube
3)Added 20 ml distilled water in each COD tube.
4) Added stones (crush on beads) 1 or 2 pieces.
5) Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
6) Added 30 ml conc sulfuric acid slowly along with swirling.
7) If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
8) Kept COD tubes in COD apparatus to digest the sample..Attached the condenser & set 150 Degree Celsius temperature.
9) Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
10) After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
11) Took the above sample in a conical flask. 12)Added 150 ml distilled water.
13) Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Performing experiment for COD determination.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
A-Reading blank solution.
B-Reading with sample solutions
N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
Result and Calculation of COD
| Sample | Readings | COD(mg/lit) |
| Blank | 9.3 | – |
| KPH | 5 | 430 |
| Sample no.1 | 12.1 | -280 |
| Sample no.2 | 7.4 | 190000. |
Conclusion :
It is very best experiment I understood different concept just like at the same time we managed all things etc.
COD is different in every sample and very good result determine in the sample of COD.
I’m pretty sured I can find COD in different water sample.
___________________________________________________________________________________________________________________________________________
25/08/2022
Lecture:
Diskshit sir gave lecture about career plans that is how to choose your career .
Work :
All 11 water beds we removed some parts of azolla and dried it.

Learned how to grow azolla, Azolla used as feed for pet and animals

___________________________________________________________________________________________________________________________________________
26/08/2022
Activity :
Preparation of different solutions for flame photometer experiment To find K (potassium) in KCL solution.
Procedure:
1)First took 0.17 gm KCL solution.
2) After in volumetric flask cantain 100ml water.(A)
3) Prepare different 5 types of solutions from solution (A) that are 10ppm,20ppm,50ppm,70ppm,100ppm.

Work:
Observed plant health and measured height and leaves size.
___________________________________________________________________________________________________________________________________________
27/08/2022
Actitvity:
Flame photometer experiment studied.Determination of Potassium in solution by flame photometer experiment.
Apparatus:
Flame photometer,small beaker,5 different types of solutions,distilled water.

Procedure :
1)Switch on the flame photometer
2)First standardized flame photometer by distill water
3)Adjusted the knob.
4) Took 5 different small beakers and pour solution on it
5)Took the reading one by one solution.
6)Note down the reading.
Result: In five different solutions we observed that are reading.
Learned graph on excel sheet.And drawn the graph Graph
Conclusion :
In this activity I learned various things just like made different type of solution how to take reading observation is most important all things I learned.
Work:
we water the plants everyday.
_____________________________________________________________________________________________________________________
28|08|2022
Sunday
___________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
29/08/2022
Activity:
Fish weight.In the fish project

1)Firstly water was removed from the tank
2)After that, all the fish were caught.
3) Then fish were weighted.
4)Fish were counted.
5) Released back into each of the 4 tanks.
6)After then fresh water was again filled in all the four tanks.

Observation:
We observed that in 4 different tanks feeding different fish diets resulted in weight gain of some fish and not in some fish and it was observed that weight gain was caused by azola given to the fish as food.

Work:
we remove all azolla fram 4 water beds and drying it under sunlight and add new fresh azolla.
______________________________________________________________________________________________________________________________________________
30/08/2022
Activity:
Plantation

1)Firstly 4 different types of soil were taken in 48 bags.
2) After that, small branches of drumstick tree were cut and planted in 24 bags
3) Also geranium plant planted in 24 bags
4) After small holes were made in each bag
5) Then the plants were watered.

____________________________________________________________________________________________________________________________________________________________________________________________________
31/08/2022
Activity:
Soil testing :
We tested the same soil where we planted the trees yesterday.
Soil testing was done as in the first activity.
Lab cleaning :
we cleaned the microbiology lab.
____________________________________________________________________________________________________________________________________________________________
1/082022.
Activity:
Chech EC,TDS,PH
learned how to checked EC,TDS,PH in different solutions.

measured Temperature and humidity in polyhouse.and noted down.
searching
different topic searched on computer.
Work:
water the plants.
2/09/2022
_____________________________________________________________________________________________________________________________________________________________________________
Holiday
__________________________________________________________________________________________________________________________________________________________________________
3\09\2022
Activity:
Preparation of H2S kit for water testing
Media preparation for 300 H2S kit strip

| name of chemicals | Quantity in gm/ml |
| Bacteriological Grade Peptone | 20 gm |
| Dipotassium Hydrogen Phosphate | 1.5gm |
| Ferric Ammonium Citrate | 0.75gm |
| Sodium Thiosulphate | 1 gm |
| L-cysteine HCl | 0.12 gm |
| Distilled water Teepol | 50 ml 1 ml |
Preparation of 50 strips
Use Whatman filter paper no-41 for the making strips of H2S Water testing kit.
Cut the paper in size of 8*2.5 cm for making 300 strips.
Dip all strips in given media which is mention in Step-1Use Hot Air oven to dry strips at 50 degree Celsius for 40 minAfter that use Laminar Air flow for sterilizing the strips.
Take 30 ml new 300 plastic bottles Sterile the bottles using Laminar Air Flow for 1 hrs.
It helps to prevent Bacterial contamination.
Making the H2S bottles with strips which is coated by H2S mediaGetting precautions for avoid the contamination70% Ethanolsterile gloves.
Use forceps for put strips into the bottles.Bottles caps should be tight.

All process are done under the UV light of Laminar Air Flow.

__________________________________________________________________________________________________________________________________________________________________________
4\09\2022
Activity
Find COD in different sample.
procedure is same as above COD activity.
packing H2S bottles.
___________________________________________________________________________________________________________________________________________________________________________
5\09\2022
Sunday
_____________________________________________________________________________________________________________________________________________________________________
06/09/2022
Firstly find information about estimation of carbon from soil sample by walky black methode.
Collect the sample that have walky black experiment.
Water the plants.
Conclusion :
Today’s I Learned theory knowledge about the experiment and it’s important for experiment.
_______________________________________________________________________________________________________________________________________________________
07\09\2022
Estimation of carbon from soil by walky black method
Introduction:-
Estimates of organic carbon are used to assess the amount of organic matter in soil . The method measures the amount of organic carbon in plant and animal remains including soil humus .
Aim :-
To estimate organic carbon from soil sample by walkey black method
Equipment:-
1. 500-mL Erlenmeyer flasks.
2. 10-mL pipette.
3. 50-mL burette.
4. Analytical balance.
5. Incandescent lamp.Reagents:1. H3PO4, 85%.2. H2SO4, concentrated (96%)3. NaCl , solid.4. Standard 0.167M K2Cr2O7:- Dissolve 4.904 g of dried (105oC) K2Cr2O7 in water and dilute to 100ml.5. 0.5M Fe2+ solution: Dissolve 49 g of Fe(NH4)2(SO4)•6H2O in 250 mL of water containing 5 mL of concentrated H2SO4 and dilute to 250 ml. The Fe2+ in this solution oxidizes slowly on exposure to air so it must be standardized against the dichromate.
6. Ferroine indicator
Procedure:
1. Weigh 1 g dried soil and transfer to a 500 mL Erlenmeyer flask.
2. Add 10 ml K2Cr2O7 solution in it.
.3. Add 20 mL of concentrated H2SO4 by means of a dispenser and swirl gently to mix of the flask out of the solution.
4. Allow to stand for 30 minutes. The flasks should be placed on an insulation pad during this time to avoid rapid heat loss.
5. Dilute the suspension with about 200 mL of water to provide a clearer suspension for viewing the endpoint.
6. Add 10 mL of 85% H3PO4 and add 0.2 g of NaCl. The H3PO4 and NaCl is added to complex Fe3+ which would interfere with the titration endpoint.
7. Add 10 drops of ferroin indicator.
8. Titrate with 0.5 M Fe2+ to a burgundy endpoint.
9. Run a reagent blank using the above procedure without soil. The blank is used to standardize the Fe2+ solution daily.

Calculations :-
For organic carbon=
C = (B-S) x M of Fe2+ x 12 x 100g soil / 4000
Where:
B = mL of Fe2+ solution used to titrate blank
S = mL of Fe2+ solution used to titrate sample
/4000 = Milliequivalent weight of C in
_________________________________________________________________________________________________________________________________________________________________________________________
08/09/2022
Find information About Total Nitrogen Determination By Kjeldahl Method.
understood the all preparation of solution how to set up and how many stage for this method.
all the write down in white board .
this method have 3 steps that are
A) Digestion
B) Distillation
C) Titration.
collected all the solution that have for experiment after set up the method.

____________________________________________________________________________________________________________________________________________________________________
9\09\2022
________________________________________________________________________________________________________________________________________________________________________
Holiday
10\09\2022
Today some visitors came to see the Vigyan Ashramand they are my Teachers. We were with them, informed them, showed them everything and interacted with them, they liked the Vigyan Ashram very much and were happy to meet us.We discussed various ideas in a group for next plane.
_______________________________________________________________________________________________________________________________________________________________________
11\09\2022
Sunday
____________________________________________________________________________________________________________________________________________________________________
12\09\2022
Activity :
Flame photometric experiment
Procedure same as above flame photometric experiment.
Removed all Azolla from 6 water bath and grow new azolla.
Water the plants .
13\09\2022
Activity
Today we learn blood group testing in food lab.
DBRT students learn blood group testing practical, that time we were went there.
We studied which antigen used for the blood group testing like as Anti-A ,Anti-B,Anti-D.
Mam taught us how to find right blood group and we learned very carefuly.

14\09\2022
Collect information about estimation of calcium and magnesium from soil sample by EDTA method.
Collect the soil dry it and weighted 25 gm of soil.
Fistly I prepared the Reagents
Reagents :-
A. Buffer Solution (NH4Cl-NH4OH) :-Dissolve 6.75 g ammonium chloride in 57 mL concentrated ammonium hydroxide, and transfer the solution to a 100ml volumetric flask, let it cool, and bring to volume with DI water.
B. Eriochrome Black Indicator
C. Ethylene diamine tetra acetic Acid Solution (EDTA):- 0.01 N Dissolve 0.2 g ethylene diamine tetra acetic acid, and 0.005 g magnesium chloride (MgCl2) in DI water, and bring to 100ml volume with DI water.
D. Sodium Hydroxide Solution (NaOH):- 2 N Dissolve 8 g sodium hydroxide in about 80 mL DI water, transfer the solution to a 100ml volume, cool, and bring to volume with DI water.
E. Ammonium Purpurate Indicator (C8H8N6O6) :-
F. Ammonium acetate solution :- Dissolve 38.5 grams of ammonium acetate in 40 ml of distilled water and bring volume up to 50 ml .
water the plants.
15\09\2022
Aim :-
To estimate calcium and magnesium from given soil sample by EDTA method
Reagents :-
A. Buffer Solution (NH4Cl-NH4OH)
B.Eriochrome Black Indicator:-
C. Ethylene diamine tetra acetic Acid Solution (EDTA):-
D. Sodium Hydroxide Solution (NaOH):-
E. Ammonium Purpurate Indicator
F. Standard Stock Calcium Chloride Solution (CaCl2.2H2O):-
G. Ammonium acetate solution :-

A . Preparation of soil extract :-
Weigh 25 grams of soil and add 100 ml of 40% ethanol to it .Filter the mixture and discard the filtrate and add ammonium acetate solution to it .Incubate the mixture overnight and use it as soil extract .

B. CALCIUM
Estimation of calcium from soil.
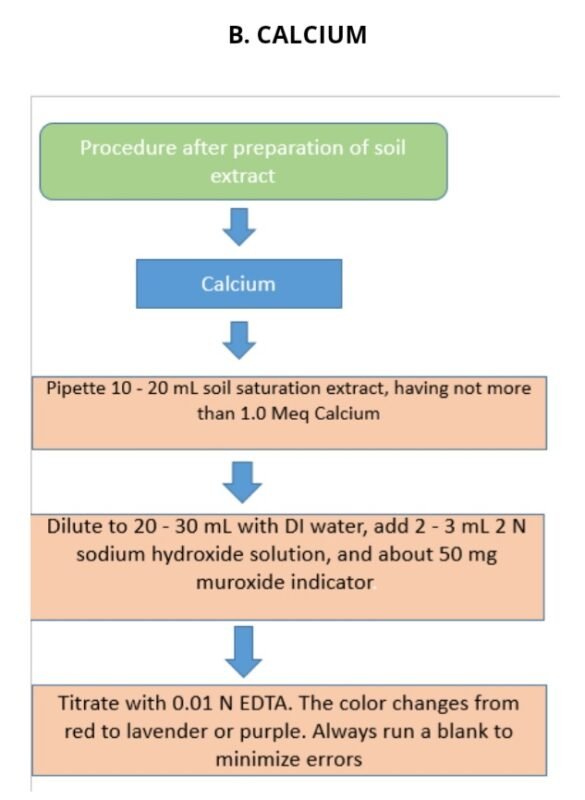

Calculations :-
Ca or Ca + Mg (meq/L) = (V – B) × N × R × 1000 / Wt of soil sample taken for mg = ( ca+mg ) – ca
Where:- V = Volume of EDTA titrated for the sample (mL)
B = Blank titration volume (mL)
R = Ratio between total volume of the extract and extract volume used for titration.
N = Normality of EDTA solution
16/09/2022
Groundnut
-Chikki Groundnut-

Chikki Roast groundnut
1. Heat a thick-bottom heavy kadai or pan and add 1 cup of peanuts and roast it .
2. Once the peanuts cool down, rub them between your palms and peel off the husks.Make Jaggery Syrup

1. jaggery block instead. Chop it finely and measure the chopped jaggery in a ½ cup measuring cup.
2. Heat the kadai (wok) or pan on a low flame. Then, use a spatula or spoon to stir the jaggery syrup until the jaggery dissolves.
3. Continue to stir the mixture continuously.
Make Peanut-Chikki
1. Once the jaggery syrup is the right consistency, add the peanuts quickly.
2. Mix the jaggery syrup and peanuts until well combined.
3.Once the peanut chikki has cooled down break and serve. If you want to serve the chikki at a later time then store it in an airtight jar at room temperature.

Date 17/09/2022
Water the plants and spraying Pesticides and required nutrients.Calcium nitrate
Date 18/09/2022
Holiday
Date 19/09/2022
Today We watering all the plants in polyhouse Observation of all plants.
Date 20/09/2022
Media Preparation & Pouring into BottlesMake Culture + Sucrose media

Requirements
:For 500 ml
Media preparation –Stock 112.5 mlStock 20.5 mlStock 32 mlStock 40.5 mlMynosyitol50 mgSucrose15 gmAgar4 gm

Procedure:
1. Take all stock solution in flask as per measurement .
2. Add 50 mg mynosyitol .
3. Add 15 gm sucrose
.4. Add Culture Mix it well .
5. check Ph of Media if Ph is less than 5.8 then maintain it by using NaOH .
6. Add 4 gm Agar .
7. Now heat the media on flame till the agar gets completely dissolved .
8. Now pour the media into bottles and autoclave it
.20/09/2022
Lab work..observed plants
21/09/2022
Find information about hardness of water.
22/09/2022
Activity Find hardness of water
23/09/2022
Holiday
24/09/2022
Azolla removing and watering all plants
Today we remove azolla and give water all the plants
25/09/2022
Holiday
26/09/2022
Find nitrogen by Kjeldahl Method Procedure same as above Activity
27/09/2022
Holiday .
Today is the last day in Vigyan Ashram, I joined on 19th August and am leaving on 3rd October. In this one and a half month period, I learned a lot of things not only through experiments but also many other things like meditation, prayers etc. Practicals in Chemistry lab are all easy to my self because of this course, and I have benefit a lot from all this in future. All the teachers were very cooperative and supportive. Whole atmosphere was pleasant. Actually I don’t want to go but having to go for the next future. Whenever I have time, I will definitely come here and learn a lot of new things. also tell more to other students. I consider this as a golden opportunity to learn many new things.




