Aim :- Estimation of calcium by using flame photometer.
Instrument required for this method :-
1)Flame Photometer

Principle of flame photometer:-
The compounds of the alkali and alkaline earth metals (Group II) dissociate into atoms when introduced into the flame. Some of these atoms further get excited to even higher levels. But these atoms are not stable at higher levels.
Hence, these atoms emit radiations when returning back to the ground state. These radiations generally lie in the visible region of the spectrum. Each of the alkali and alkaline earth metals has a specific wavelength.
| Element | Emitted wavelength | Flame color |
|---|---|---|
| Sodium | 589 nm | Yellow |
| Potassium | 766 nm | Violet |
| Barium | 554 nm | Lime green |
| Calcium | 622 nm | Orange |
| Lithium | 670 nm | Red |
For certain concentration ranges,
The intensity of the emission is directly proportional to the number of atoms returning to the ground state. And the light emitted is in turn proportional to the concentration of the sample.
Chemicals required for this method:-
1)Calcium carbonate.(CaCO3)
2)Hydrochloric acid.(conc.HCl)
Preparation of Solutions:-
1)Standard Preparation (CaCO3 solution)
Place 1.249gm AR Calcium Carbonate in approximately 50ml H2O.Adding dropwise conc. HCl until calcium carbonate is dissolved (should take about 10ml ).
Procedure:-
By using CaCO3 we prepared stock solution which have 1249 mg in 50 ml distilled water. By using this stock solution prepared different standard solutions as follow:
Standard curve with different conc. of CaCO3 solution by using flame photometer.

Checked absorbance of above solutions by using flame photometer as follow:

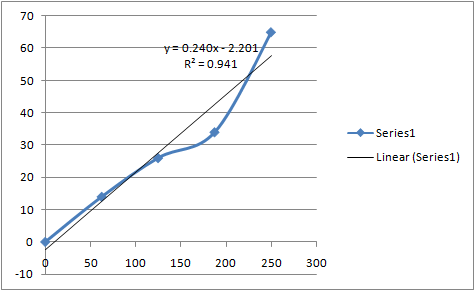
| conc. (mg) | Absorbance |
| 0 | 0 |
| 62.46 | 14 |
| 124.9 | 26 |
| 187.35 | 34 |
| 249.8 | 65 |
Standard graph of Calcium Concentration vs Absorbance:-
Unknown sample absorbance reading :-
| Sample name | CaCO3 Unknown sample | Tap Water | Vermiwash | Hydroponics |
| Absorbance | 20 | 03 | 36 | 04 |
Calculations :-
Used equation – y= mx+c

Results:
| Sample Name | Unknown CaCO3 sol. | Tapwater | Vermiwash | Hydroponics |
| mg of Calcium | 92.42mg/l | 21.6 mg/l | 159.03 mg/l | 25.81 mg/l |
Flame View of Calcium On Flame Photometer:-











