what is ammonia?
Ammonia is a nutrient that contains nitrogen and hydrogen. Its chemical formula is NH3 in the un-ionized state and NH4 + in the ionized form. Total ammonia is the sum of both NH3 and NH4 + . Total ammonia is what is measured analytically in water.
Introduction:_
Ammonia is also one of the most important pollutants because it is relatively common but can be toxic, causing lower reproduction and growth, or death. The neutral, un- ionized form (NH3 ) is highly toxic to fish and other aquatic life.
Ammonia is the preferred nitrogen-containing nutrient for plant growth. Ammonia can be converted to nitrite (NO2 ) and nitrate (NO3) by bacteria, and then used by plants. Nitrate and ammonia are the most common forms of nitrogen in aquatic systems. Nitrate predominates in unpolluted waters. Nitrogen can be an important factor controlling algal growth when other nutrients, such as phosphate, are abundant. If phosphate is not abundant it may limit algal growth rather than nitrogen. Ammonia is excreted by animals and produced during decomposition of plants and animals, thus returning nitrogen to the aquatic system.
Need of this project:-
I had decided to work on this project,because fish mortality rate is gradually increases in aquaponics of vigyan ashram due to increase ammonia level in water.so I choose to do this project due to need of estimation of ammonia to solve this problem.
Aim:-Estimation of percentage of ammonia by using kjeldahl process.
I studied different method for estimation of ammonia in water and organic compound but here I selected one of the method for estimation of ammonia are as follow,
Diagrammatic representation of kjeldiahl set up:-
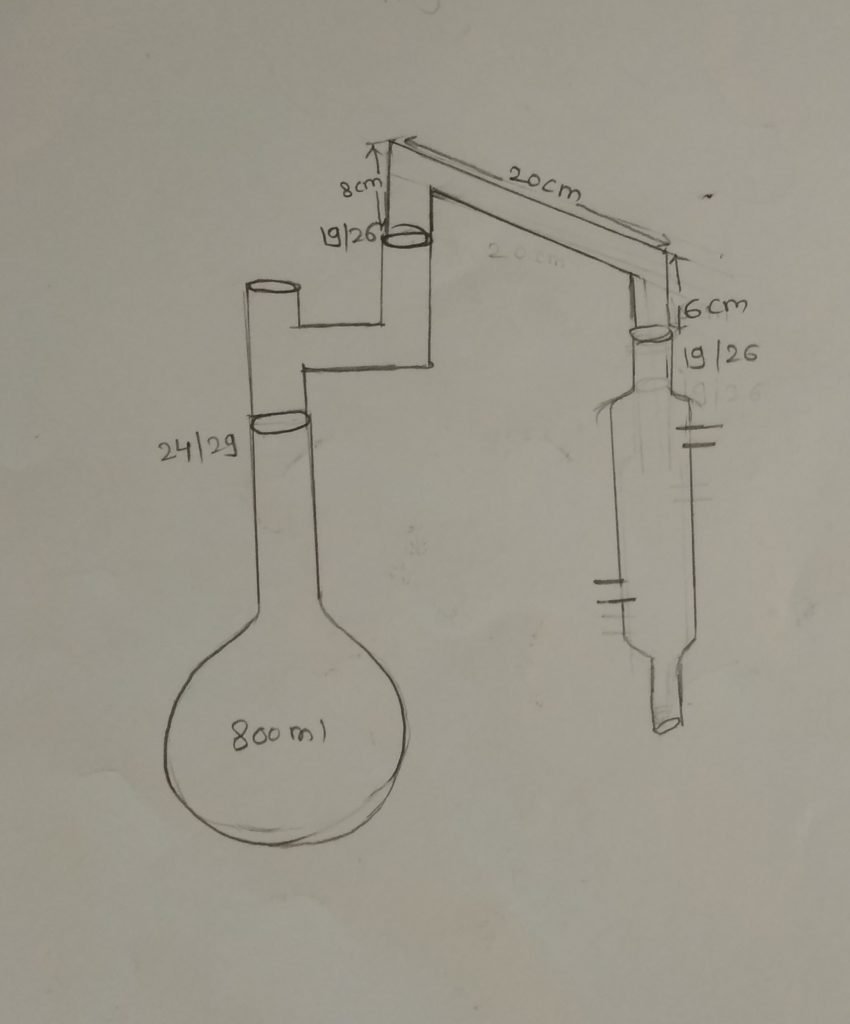
Apparatus:–
Digestion tube, Erlenmeyer flask , burette, Condenser, Heater.
Chemicals:-
Potassium Sulphate(K2So4), Conc. H2So4(Sulphuric acid), 35% NaOH, 4%Boric Acid(H3Bo3), 0.1MHCl, Methyl orange indicator
Procedure:
-This process involves into three steps:
1)Digestion
2Distillastion
3)Titration
*Digestion:
1)Taken appro. 10 ml of water sample from fish tank in digestion tube.
2)Added appro. 5 gm of K2So4 with hydrous cupric sulphate(CuSo4.5H2O) catalyst in digestion tube
3)Added 20ml H2So4(98%) in digestion tube
4)Heat the mix. continuosly at 350-380 degree celcius.
5)continued heating for about 180 minute.
6)Sample cooled at room temp.
*Distillastion
1)50ml of NaOH(50%) sol. is added to the sample to neutralised
2)NH3 is condensed
3)NH3 is captured in a 50 ml Boric acid
4)Around 150 ml of condensate is captured in the boric acid sol.

*Titration
Titrate above sample with HCl (0.1molar) until sol. has slightly violet colour

.
*Conclusion:-Many times I worked on this process but some faults are found results are also not found so I decided apply another process this method as follow.
Result not Found ,so had proceed for another method
Estimation percentage of ammonia by using colorimeter & kjeldahl method -1:-
Above procedure failed then I used combined procedure of colorimeter & kjeldahl method .
Equipments required for this method:-
1)Colorimeter- 115:- It is required to measure the absorbance of solutions.

2)Heater:- It is required to heat the sample in distillation process.

3)Electric Motor:-

Glassware required for this method :-
1)Digestion Flask (800ml):-

2)Condenser:-

3) Erlenmeyer flask :-

4)Joints :-

Procedure:- 1st I prepared solutions – Nessler reagent, EDTA, Ammonium sulphate,
preparation of solution
1)Nessler reagent:-
10gm HgCl2 (mercuric chloride) , 7gm KI(potassium iodide), 16gm NaOH (sodium Hydroxide) are dissolved in 100ml distilled water .
Kept above solution 5 days in dark place after 5 days used this solution.
2)EDTA
39.2gm EDTA, 20.72gm NaOH , dissolved in 100 ml distilled water.
3)Ammonium Sulphate solution
Dry 1gm ammonium sulphate in oven at 100 degree celcius for 1 hour
After accurately weight 0.4718 gm of ammonium sulphate and dissolved in 100 ml distilled water . again take 1 ml solution from this solution and dissolved in 100ml distilled water.
4)NaOH solution
Dissolved 24 gm sodium hydroxide pelletes in 100 ml distilled water
5)Zinc sulphate heptahydrate solution:-
Dissolved 10 gm zinc sulphate heptahydrate in 100 ml distilled water
*Procedure on colorimeter
1st on switch of colorimeter
1). systronics colorimeter 115
2) Keep sample Holder empty
3)Busy
4)Measuring mode
5)After this take Absorbance
6)Put sample & enter
7)Busy
8)Take reading
For ammonia test need to draw graph for this take readings of given chemical
prepared solution by using following table and take absorbance on colorimeter.

Readings:-Taken absorbance on colorimeter
| sr no | concentration(ml) | Absorbance(at 420nm) |
| 1) | 0 | 0.168 |
| 2) | 1 | 0.225 |
| 3) | 3 | 0.284 |
| 4) | 5 | 0.336 |
| 5) | 8 | 0.403 |
| 6) | 10 | 0.312 |
*#*For estimation of ammonia I followed combine process of colorimeter & kjeldiahl method. In this process nesseler reagent act as main role in first step and in second step NaOH act as main role.
Chemicals:- Nessler reagent, NaOH (50%), Boric acid,Methyl red indicator.
*Procedure:-
1st step:-
- Taken 49 ml test water sample ,added 1ml Nessler reagent in a beaker.
- Allow the beaker to stand for 20 minute.
2nd step
1)Taken above solution in digestion flask due to cause of distillation.
2)Added 50 ml NaOH(50%) in above solution.then sample neutralised.
3)Heated above solution .
4)A stream of water vapour is bubbled into the sample to entrain the NH3 formed.
4)NH3 is condensed.
5)NH3 is captured in a 50 ml of Boric acid sol. (4%) that contain 6-7 drop of methyl indicator.
6)When NH3 react with boric acid the the solution turns from red violet to to green due to the color change of the indicator from acid to basic medium.
7)Around 150 ml of condensate is captured in the boric acid solution.
*Titration:
Titrate above solution with 0.1 molar HCl solution until solution has slightly violet colour.
Here I have end point not found during titration color not changes .
.Conclusion:-
In this process I found some fault so this process stop here & I use another method.
Estimation Of percentage of ammonia by using combined process of colorimeter & kjeldiahl process :-In above method some equipment problem are found thats why above method can’t apply for solving this problem I do some modification and changes in current set up this changes as follow.
Difficulties:-During experiment we have to care about apparatus because following problem are found ,
During distillation the condensate reverse back in digestion flask solution temperature difference between condensate and digestion flask solution thats why apparatus (digestion flask) cracked.

For preventing this problem following precaution we take
1)we have to need to give proper insulation.
2)We have to maintain low heat.
3) We have to do Some small changes in set up.
Aim :- Estimation of percentage of ammonia by using colorimeter & kjeldiahl process
I do some changes in following experimental set up .used proper insulation for distillation set up as follow.

Chemicals required for this method:–
1)Ammonium Sulphate (NH4)2SO4.
2)Nessler Reagent
3)NaOH (40%)
4) HCL (0.1M)
Preparation Of Chemicals:-
1)Ammonium Sulphate:
weight accurately 1 gm ammonium sulfate (NH4)2SO4 & dry for 1 hour in oven . Then accurately weight 0.4718 gm and dissolved in 100ml distilled water (stock solution) .Take again 1ml (4.718mg) & dilute for 100 ml (standard solution).
2)Nessler reagent:-
10gm HgI + 7 gm KI +16 gm NaOH dissolved in 100 ml distilled water.
3) NaOH (40%):-
Take 40 gm NaOH pelletes dissolved in some distilled water & make up 100 ml distilled water.
4)HCL (0.1M):-
Take 8.33ml Conc HCl dilute for 1000ml distilled water.
Procedure:-
This method divided into two steps
1)Distillation 2)colorimetric determination.
1)Distillation :-
Preparation of equipment:-
1)Add 100ml distilled water to an 800 ml kjeldahl flask. & then add 5ml ammonium sulphate solution as sample (standard sample 25 mg ammonium sulphate) .
2)Add 3 ml sodium hydroxide (NaOH) (40%) .Kept kjeldahl flask on heater & switch on of heater at 80 degree celcius. Here distillation process start .
3)Captured above distillate in 0.1 M HCl (50ml) .Captured approximately 100 ml distillate with conical flask solution.
Colorimeteric Determination :-
1)Prepare a series of Nessler tube standards as follows :
ml of Standard
| 1.0ml=mg NH3-N | 0.0 | 0.5 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 8.0 | 10.0 |
| mg NH3-N/50.0 ml | 0.0 | 0.005 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.08 | 0.10 |
2) Dilute each solution to 50 ml with distilled water,& then add 2 ml of Nessler reagent and mix.

3)After 20 minutes read the absorbance at 420 nm against the blank on colorimeter.
4)From the values obtained plot absorbance vs. mg NH3-N for the standard curve.
5)Determine the ammonia in the distillate by nesslerizing 50 ml and reading the absorbance at 420 nm as described above for the standards.

Standard curve by using standard solution of ammonium sulphate (NH4)2SO4 (4718 ppm):- 1st I prepared 4718 ppm stock solution of (NH4)2SO4 and then dilute this stock solution in different standard solutions.like following tables.
1st Standard curve :-
| Concentration(ml) | 0 | 0.5 | 1 | 2 | 3 | 4 | 5 | 8 | 10 |
| Absorbance(420nm) | 0.071 | 0.082 | 0.107 | 0.078 | 0.131 | 0.137 | 0.195 | 0.214 | 0.228 |
Graph of absorbance vs Concentration

2nd Standard curve:-some calculations for preparation of solutions
0.4718gm of ammonium sulphate (NH4)2SO4 in 100ml distilled water.
471.8 mg (NH4)2SO4 = 100ml
4.718 mg (NH4)2SO4 =1ml
by using 4.718mg of ammonium sulphate ( standard sol) I prepared different solutions and check absorbance by using colorimeter.and then I got following graph.
Readings of given sample by using colorimeter:–
| concentration(ml) | 0 | 0.5 | 1 | 2 | 3 | 4 | 5 | 8 | 10 |
| Absorbance(420nm) | 0.031 | 0.027 | 0.031 | 0.068 | 0.071 | 0.101 | 0.108 | 0.164 | 0.186 |
Standard curve of above readings:–

| concentration (ppm) | Absorbance |
| 0 | 0.063 |
| 2 | 0.108 |
| 4 | 0.178 |
| 6 | 0.188 |
| 8 | 0.212 |
| 10 | 0.237 |
Standard Curve :-
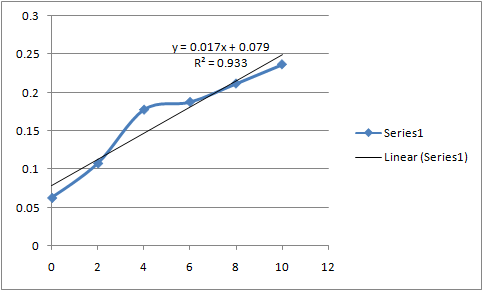
Standard curve :-
| Concentration (ppm) | Absorbance |
| 0 | 0.093 |
| 3 | 0.166 |
| 6 | 0.227 |
| 9 | 0.266 |
| 12 | 0.323 |
| 15 | 0.405 |
Standard Curve Absorbance Vs Concentration
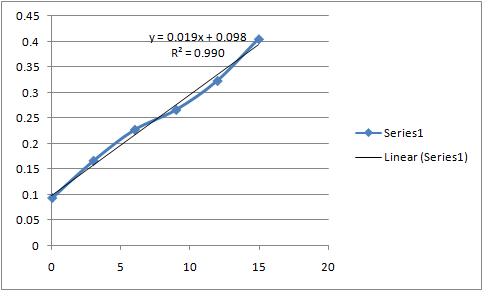
Readings :-
| Cocentration(ppm) | Absorbance |
| 0 | 0.112 |
| 1 | 0.118 |
| 3 | 0.173 |
| 5 | 0.203 |
| 7 | 0.257 |
| 9 | 0.290 |

Conclusion :- All above standard curve 1,2,3,4,5 indicate prepared solutions are correct. R^2 value also good.So I decided use this method for estimation of ammonia.
Method :-