Introduction: Dissolved oxygen (DO) is essential for maintaining healthy water bodies. It is the amount of oxygen dissolved in unit volume of water. Dissolved Oxygen depends on physiology and biochemical activities in water body. Dissolved oxygen refers to the amount of oxygen present in water. It is essential for aquatic life, aquatic plants and aquatic animals. Hydroponic is a method growing plants without soil, using mineral nutrients solutions. Dissolved Oxygen required for growth of roots in plants, roots of plants to absorb nutrients efficiently , roots to carry out respiration. Adequate amount of dissolved oxygen help to prevent root rot and promote healthy plant growth in hydroponic system. The standard recommended range for DO between 5-8 mg/L. This range ensure the roots of plant absorb nutrients efficiently which is crucial for overall health and productivity of plants. If the range falls below the range result in reduced nutrients uptake by the plants, roots suffocation and increased susceptibility to root disease. While excess dissolved oxygen leads to photosynthesis inhibition, Nutrients imbalance, Root problem, stress and reduced growth.
When water holds as many dissolved gas molecules as it can, this is known as 100% saturation. It is possible for saturation to exceed 100%, and this is known as super-saturation.Most greenhouse crops will perform better with higher DO levels, with levels greater than 8mg/L generally being considered as good for greenhouse production. Research has also found that having a high concentrated dissolved oxygen (20-30 mg/L) supply was effective in improving plant growth under a low greenhouse temperature in a deep hydroponic culture. However, if dissolved oxygen levels drop below 4 mg/L, the water is considered to be hypoxic which is detrimental to plants. Dissolved oxygen levels below 0.5 mg/L is considered to be anoxic and does not support plant or animal life.
Two Methods commonly used to determine DO
- Iodometric Method- Titration based method depends on oxidising property of DO
- Membrane Electrode- Based on rate of diffusion of molecular Oxygen across the membrane.
Iodometric Method-
Principle:- In alkaline medium DO oxidizes manganese sulphate to insoluble brown precipitate of hydrated manganese dihydroxide
MnSo4 + 2 KOH → Mn(OH)2 + K2SO4
2 Mn(OH)2 + O2 → 2 MnO(OH)2 (s) Brown ppt
MnO(OH)2 dissloves in conc. H2SO4 , In presence of iodide ions in an acidic conditions the oxidised manganese revert back to the diavalent state with liberation of iodine equivalent of the original DO content.
MnO(OH)2 + 2 H2SO4 → Mn(SO4)2+ 6 H2O
Mn(SO4)2 + 2 KI → MnSO4 + I2 + K2SO4
MnO2 + 4 H+ + 2 I → Mn 2+ + I2 + 2 H2O
The liberated iodine is estimated by standard sodium thiosulphate (Na2S2O3) solution by using starch as indicator.
2 Na2S2O3 + I2 → Na2S4O6 + 2 NaI
Hence, 2 Na2S2O3 = I2 = MnO2 = 1/2 O2
Standardization of Sodium Thiosulphate (Na2S2O3 )
Sodium Thiosulphate (Na2S2O3)is secondary standard and is standardized by Potassium Dichromate (K2Cr2O7) Using starch indicator.
Apparatus:- Burette, Burette stand, conical flask, reagent bottle, measuring cylinder, Dropper etc.
Chemicals:- Potassium Dichromate (K2Cr2O7), Sodium Thiosulphate (Na2S2O3), Potassium Iodide(KI), conc. HCl, KOH/NaOH (Sodium/ Potassium Hydroxide), Sodium Azide (NaN3), conc. H2SO4 , Starch.
Preparation of Reagents:-
- Preparation of 250ml 0.05N Na2S2O3. 5H2O Equivalent Weight = Molecular Weight/ Valance factor =[23×2+32×2+16×3+5×2+16×5]÷1= 248g Mass of solute = Normality × Equivalent Weight × Volume of solution = 0.05×248×0.250 = 3.1g. 3.1g dissolved into 250ml Distilled water.
- Preparation of 250ml 0.05N K2Cr2O7 Gram Equivalent Weight = 39×2+52×2+16×7÷6= 49g Normality = Mass/Equivalent Weight × volume Mass = 0.05×49×0.250 = 0.613g 0.613g dissolved into 250ml of distilled water
- Preparation of 50ml Alkaline-Iodide-Azide Solution. 35g KOH, 7.5g KI, 0.5g NaN3 dissolved into distilled water and make upto 50ml.
- Preparation of Manganese Sulfate MnSO4. H2O. 18.2g MnSO4. H2O dissolved in 50ml distilled water.
- 1% Starch Solution:- 1g starch dissolved in 100ml distilled water and boiled for few min until clear solution is appeared.
Procedure:-
Standardization of 0.05N (Na2SO4)Sodium Thiosulphate by standard 0.05N Potassium Dichromate (K2Cr2O7)
- Take 10ml of 0.05N Potassium Dichromate in conical flask and add 0.8g of potassium iodide (KI) into the flask shake it to dissolve.
- Add 1ml conc. H2SO4 and kept the flask in dark for 10 min.
- Titrate it against 0.05N Sodium Thiosulphate (Na2S2O3) using starch indicator until blue colour disappear.
- Record the readings.
Titration with Water Sample
- Collect the sample without bubbling in 300ml BOD bottle.
- Add 2ml of Manganese sulfate (MnSO4. H2O) solution inserting the pipette tip into the sample because drop of solution can allow inserting the oxygen into sample.
- Add 2ml of Alkali-Iodide-Azide reagent by above method. Allow reacting the solution with oxygen present in sample.
- when precipitate settle down at the bottom add 2ml of Sulfuric Acid H2SO4 by placing the pipette very near to sample surface.
- Mix well to dissolve the precipitate.
- Take 100ml sample in the flask and titrate it against Sodium Thiosulphate using starch indicator until blue colour disappear.
Observations
- Standardization of sodium thiosulphate

⬇️



2. Estimation of dissolved oxygen in water






Observation Table –
- Standardization of Na2S2O3 by K2Cr2O7

2. Estimation of dissolved oxygen in water of Tank B

N1V1 = N2V2
N1* 50 = 0.058 * 0.96
N1= 0.058* 0.96/ 50 = 0.0011N
dissolved oxygen = Normality * Equivalent Weight = 0.0011* 4 = 0.0044g/L = 4.4mg/L
3. Estimation of dissolved oxygen in water Tank C

Dissolved Oxygen = Normality * Equivalent weight = 0.0014*4 = 0.0056g/L =5.6mg/L
4. estimation of dissolved oxygen in water tank NFT
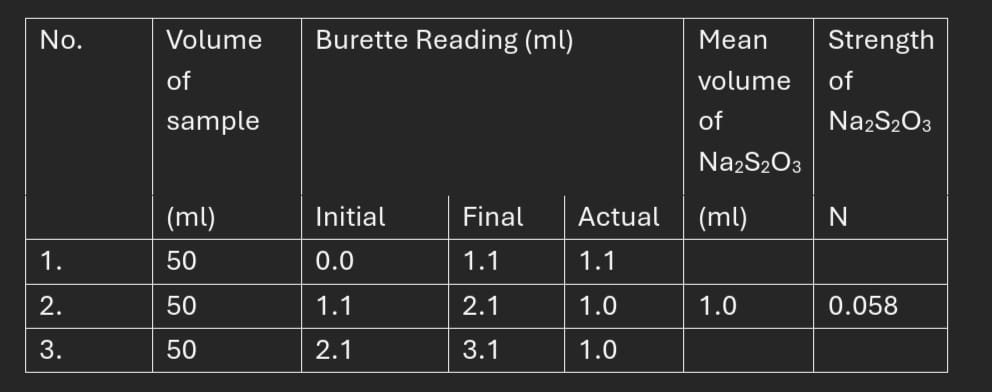
Dissolved Oxygen = Normality * Equivalent weight
=0.00118* 4
= 0.00472g/L = 4.72mg/L
Result :- dissolved oxygen from hydroponic water tank
- Tank A – 0mg/L ( No ppt was appeared after addition of MgSO4 and alkaline-azide reagent, instead of brown ppt white ppt was observed )
- Tank C – 5.6mg/L
- Tank NFT – 4.72mg/L
- Tank B – 4.4mg/L
Conclusion :- Tank A with without bubbler system while Tank B, Tank C and Tank NFT ( Nutrient Flow Tank) were having bubbers to increase the Dissolved Oxygen from tank. As from above result we can see that tank B,C and NFT with the Do range close to the permeable limit.
7 December 2024
I had talk with Dixit Sir about what gonna the work should have to be done in this project.
2 January 2024
After this decided to vary the temperature and observe the change in DO level
recorded the DO level of tap water by varying temperature with zero DO , 10, 20, 30, 40 and 50 degree Celsius.
Procedure :-
- physical techniques to remove DO from water, we used Thermal Degassing in that water boiled water at 1atm. water has placed in cooker and 2-3 whistle taken, the water in the flask slowly poured into plastic bottle. After it cooled, calculated the DO by using winklers method.
- Ice cubes are used to decrease the temperature, DO calculated by titration method
- To increase the Temperature water heater was used and as above method DO was calculated
- First we tried with Tap water.
Observations:-

DO performed with Tap Water :-
| Sr No. | Temperature (o C) | Observed DO (mg/L) |
| 1. | 10 | 4.33 |
| 2. | 20 | 3.86 |
| 3. | 30 | 3.50 |
| 4. | 40 | 3.28 |
| 5. | 50 | 3.03 |
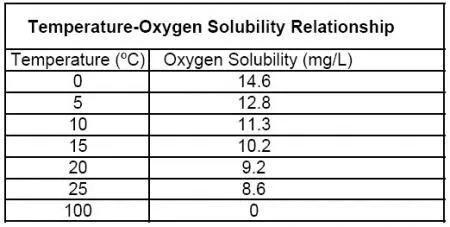
The Above table is a reference table.
The above chart shows the relationship between temperature and oxygen solubility if temperature increases the dissolved oxygen also decreases, they are inversely proportional to each other. But in our case there is no such change observed. this might be due to high TDS(Total Dissolved Solids) level in water.
4 January 2024
Then we decided to work with distilled water for to avoid that TDS factor. The same procedure has done to calculation part and for titration. For oxygen source we used air bubbler.
Observation Table – For Distilled Water
| Sr No. | Temperature (o C) | Observed DO (mg/L) |
| 1. | 10 | 5.56 |
| 2. | 20 | 5.50 |
| 3. | 30 | 4.80 |
| 4. | 40 | 4.07 |
| 5. | 50 | 3.98 |
Even through we performed with distilled water and for oxygen source air bubbler did not get the results.
10 January 2024
Oxygen Enhancer Tablets are used to increase the dissolved oxygen in water at room temp.
Below table shows the after addition of oxygen tablet the Do increase till it’s supersaturation point.
Table DO Oxygen Enhancer Tablet :-
| Sr No. | Water Type | DO (mg/ml) |
| 1. | Tap Water | 9.80 |
| 2. | Distilled water | 11.7 |