Introduction
Plant tissue culture is a scientific method used to grow plant cells, tissues, or organs under sterile and controlled laboratory conditions. The process uses a nutrient-rich medium that supports plant growth.
The main principle behind this technique is “Totipotency” — the ability of a single plant cell to regenerate into a complete plant.
The idea of tissue culture was first introduced by Gottlieb Haberlandt, known as the Father of Plant Tissue Culture.
Why Tissue Culture for Banana
Banana is one of the most widely grown fruit crops. Tissue culture is especially useful for banana propagation because it helps in producing healthy, uniform, and disease-free plants in a short time.
Advantages of Plant Tissue Culture
- Mass Production: Produces a large number of plants in a short time — ideal for commercial use.
- Disease-Free Plants: Helps eliminate harmful microorganisms like bacteria, fungi, and viruses.
- Conservation of Rare Varieties: Useful for saving valuable or endangered plant species.
- Faster Growth: Plants grow more quickly than in traditional propagation methods.
- Genetic Uniformity: All plants produced are clones, maintaining uniform quality and yield.
Stages of Banana Tissue Culture
- Initiation:
Healthy plant tissue (called an explant) is collected from a mother plant and sterilized. - Establishment:
The explant is placed in a sterile nutrient medium containing essential nutrients and plant hormones. - Multiplication:
The explant starts developing multiple shoots or callus tissue. - Differentiation:
Callus tissue begins forming into specific plant parts like shoots or roots. - Shooting & Rooting:
Shoots and roots develop on the medium. - Acclimatization (Hardening):
The plantlets are gradually exposed to external conditions like light, humidity, and temperature before being transferred to the nursery or greenhouse.
14 Dec 2025
The project was initially assigned to Amisha. After the successful completion of eight stages of banana tissue culture during her fellowship period, I took over the project and continued the mass production of banana plant tissue culture (PTC) by following the standard operating procedure (SOP) for banana.
The primary objective of this project is to achieve 100% reduction in microbial contamination in plant tissue culture. The work is being continued with the aim of successfully producing 100 banana plants under sterile tissue culture conditions.
This project was assigned to me by Dr. Dixit Sir on 14 December. During this project, Amisha is providing hands-on training, including how to use the autoclave and hot air oven, pH adjustment, preparation of MS medium, and cleaning of contaminated glass bottles.
MATERIALS:-
| Petri Plates | Tissue paper |
| Test Tube | Distilled water |
| Test Tube Stand | Sterile Distilled water |
| Beaker | Sterile Surgical Blades |
| Culture Bottle | Autoclave Bag |
| Scissors | Filter paper |
| Spatula | Forecep |
| Scalpel | Conical flask |
| Cotton plug | Measuring Cylinder |
CHEMICALS FOR MS MEDIA:-
| Macronutrients |
| Micronutrients |
| Vitamines |
| Hormones |
| Agar powder |
| Iron source |
| Amino acid |
| NaOH |
| HCl |
CHEMICALS USE FOR STERILIZATION:-
| Ethanol | Bavistin |
| Savlon ( Dettol) | Antibiotic |
| Tween 20 (liquid soap) | Sodium hypochlorite |
PREPRATION OF MACRONUTRIENTS SOLUTION:-
| Macro nutrients | Original Concentration | Amount of salt after multiplying original concentration by 20X, Solution Volume (250ml) |
| NH4NO3 | 1650mg/l | 8250mg |
| KNO3 | 1900mg/l | 9500mg |
| KH2PO4 | 170mg/l | 850mg |
| MgSO4.7H2O | 370mg/l | 1850mg |
| CaCl2.2H2O | 440mg/l | 2200mg |
| CaCl2(fused) | 330mg/l | 1650mg |
PREPARATION OF VITAMINS:-
| Vitamins | Original Concentration | Original Concentration multiplying by 1000X, Final solution volume (50ml) |
| Thiamine HCl | 0.1mg/l | 5mg |
| Pyridoxin HCl | 0.5mg/l | 25mg |
| Niacin | 0.5mg/l | 25mg |
| Glycine | 2.0mg/l | 100mg |
IRON SOURCES:-
| Sources | Original Concentration | Original Concentration multiplying by 400X, Final solution volume (50ml) |
| FeSO4.7H2O | 27.8mg/l | 2780mg |
| Na2EDTA.2H2O | 37.3mg/l | 3730mg |
MESOINSITOL:- When we prepare 1 litre ms media then we will take 100mg mesoinositol.
SUCROSE (3%):-When we prepare 1 litre ms media then we will take 30gm sucrose.
Preparation of ms (Murashige and Skoog ) media:-
| Materials | Final Volume (500ml) | Final Volume (250ml) |
| Distilled water | 250ml | 100ml |
| 20X, Macronutrients | 25ml | 12.5ml |
| 100X, Micronutrients | 1.25ml | 0.625ml |
| 1000X, Vitamins | 1.25ml | 0.625ml |
| 400X, Iron sources | 0.5ml | 0.25ml |
| Mesoinositol | 50mg | 25mg |
| Sucrose | 15gm | 7.5gm |
| Agar powder | 4gm | 2gm |
PREPARATION OF HORMONES:-(Cytokinin & Auxin Solution Preparation)
BAP Stock:-
100mg BAP in conical flask (250ml) dissolve with ethanol or 1M NaOH solution and BAP powder dissolve completely in solution and make final volume 100ml solution with Distilled water.
IBA Stock:- 100mg of IBA in conical flask (250) dissolve with ethanol or 1M NaOH solution and IBA powder dissolve completely in solution and make final volume to 100ml with Distilled water.
pH CHECK:- Prepare solution media pH range is 5.8 – 5.9 by using HCl or 1M NaOH.


Agar:- Take 8 gm agar powder and add in media ,mix with glass rod then in microwave the media for 14 minutes and agar dissolve completely in media.
STERILIZATION PROCESS :-
• First, we have cleaned glasswares such as test tube, Petri plates, conical flask, beaker, measuring cylinder, forecep, spatula , scalpel, surgical blades etc.Then, the cleaned items were dried in a hot air oven at180°C for 10 to 12 hours to ensure no water droplets remained. Then we have wrapped glassware in autoclave bag then put in autoclave with distilled water and culture media.
• Autoclave work at high pressure and temperature ( 15 psi, 121°C) for 15 to 20 minutes and kill the microorganisms. After sterilization removed glassware from sterilizer. And placed in laminar air flow then when we use it, we will turn on UV – ray light for 1 hour.
15 Dec 2025 – 22 Dec 2025
METHODOLOGY:-
- Select mother plant which age is 6-12 olds and do not any disease and high yield desirable traits.
- Cut the mother plant in desirable shape and placed dry tray.
- Wash the cut explant in running water for 15 minutes.

4. Handle the plant material with sterile gloves .
5. Then prepare total volume 300ml soap solution ( 30ml soap liquid and 270ml distilled water).
6. Wear glove hand and wash the plant material for 5 minutes.
7.Then remove the soap solution and placed in glass beaker.


8. Cut the material desirable size with the help of knife and remove the upper cover of material.
9. Then soak the material in savlon solution ( 3 ml savlon and 300 ml distilled water) for 15 minutes.
10. Then wash the plant material from sterile distilled water 3 to 5 time in bottle beaker.
11. Prepare 1% sodium hypochlorite solution and taking sodium hypochlorite solution into a laminar air flow and soak the plant material in sodium hypochlorite solution for 15 minutes( for 1 jar 50ml sodium hypochlorite and 250ml sterile distilled water).
12. After 15 minutes then remove plant material from sodium hypochlorite solution and wash the plant material 3 to 5 times in sterile distilled water.
13. Then cut the plant material required culture bottle size with the help of knife on sterilized brown paper.


14. Prepare antibiotic solution using Cefotaxime antibiotic( 200ul antibiotic and 200ml sterile distilled water) and soak the plant material in this solution for 20 minutes. Using only fresh antibiotic solution.


15. Then inoculate the plant material in culture medium( bottle beaker) and remove laminar air flow placed inoculate culture bottle at 24°C with photo periods of 6 to 7 hours light and 8 hours darkness for tissue culture sterile environment.
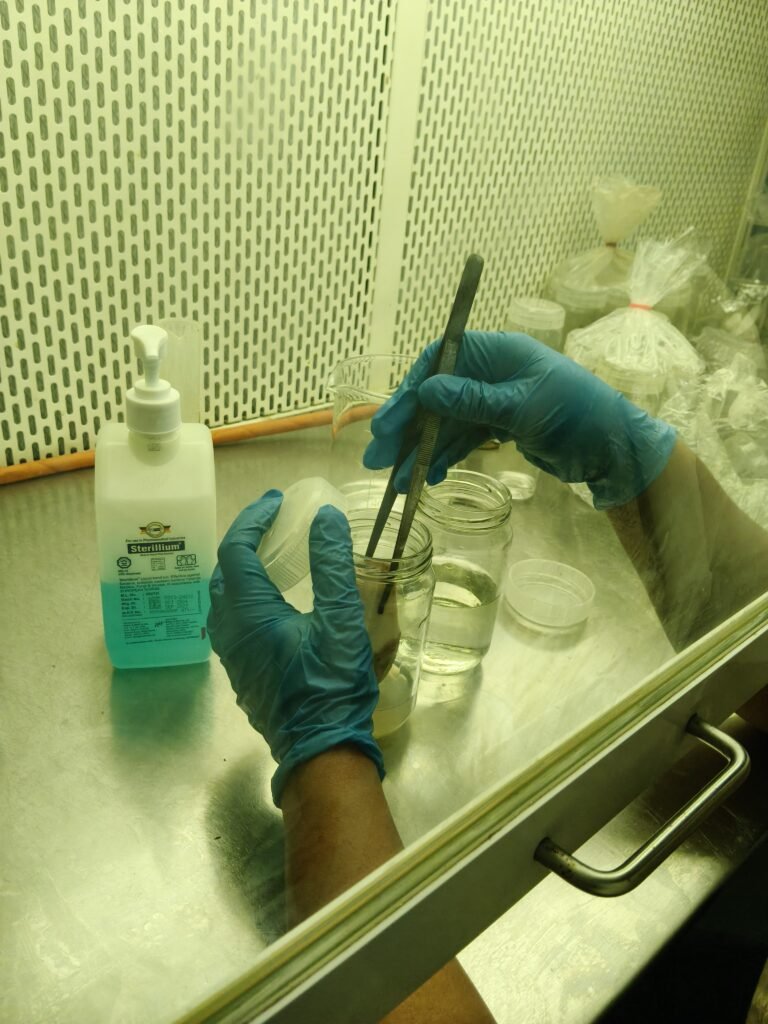

Observation : This week, plant tissue culture of banana was carried out in three bottles, and the bottles are currently under observation.
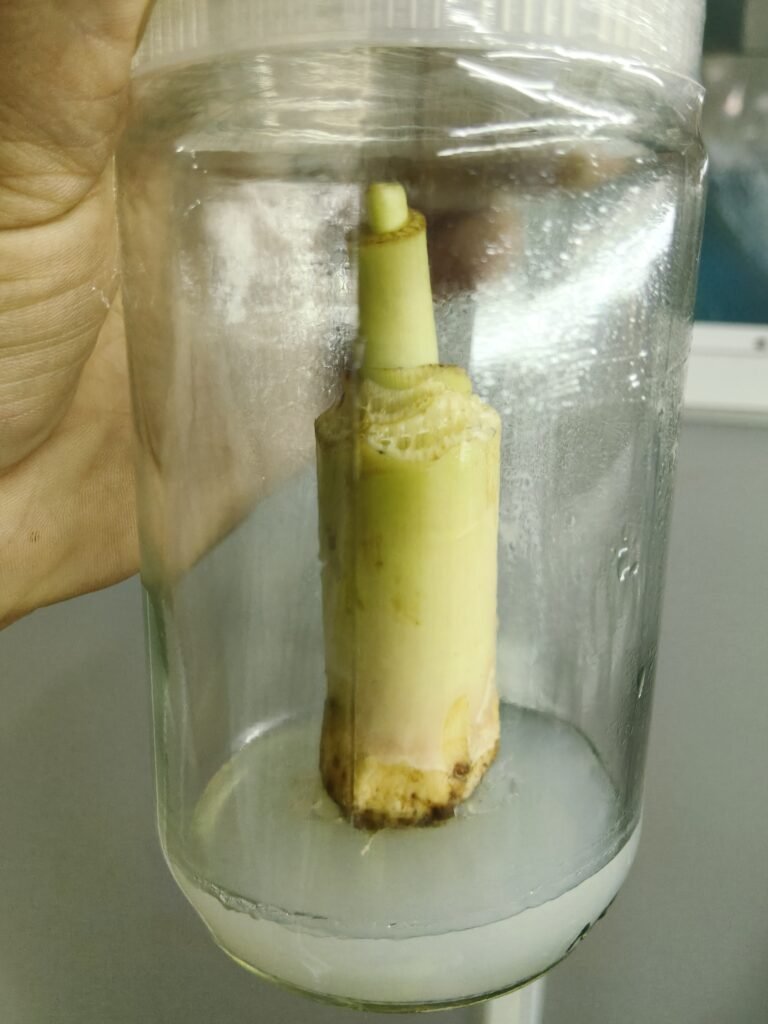
23 Dec – 24 Dec 2025
2 healthy banana sucker explants were collected from the field. The explants were thoroughly washed and surface sterilized using appropriate sterilizing agents. After rinsing with sterile distilled water, the explants were inoculated onto Murashige and Skoog (MS) medium under aseptic conditions inside a laminar airflow cabinet.
28 Dec 2025
Preparation of Macronutrient Stock Solution (250 ml), which is used for MS medium in plant tissue culture.
| Chemical Name | Chemical Formula | Original Concentration (mg/L) | Required Amount for 20× in 250 mL (mg) | Required Amount (g) |
|---|---|---|---|---|
| Ammonium nitrate | NH₄NO₃ | 1650 mg/L | 8250 mg | 8.25 g |
| Potassium nitrate | KNO₃ | 1900 mg/L | 9500 mg | 9.50 g |
| Monopotassium phosphate | KH₂PO₄ | 170 mg/L | 850 mg | 0.85 g |
| Magnesium sulfate heptahydrate | MgSO₄·7H₂O | 370 mg/L | 1850 mg | 1.85 g |
| Calcium chloride dihydrate | CaCl₂·2H₂O | 440 mg/L | 2200 mg | 2.20 g |
| Calcium chloride (fused / anhydrous) | CaCl₂ (fused) | 330 mg/L | 1650 mg | 1.65 g |

30 Dec 2025 – 2 Jan 2026
Today, I prepared 10 bottles of hormone-MS media, which will be used for 5 bottles of banana plant tissue culture maintained at Stage II.
| Material Name | Final Volume (for 250 mL) |
|---|---|
| Distilled Water | 100 mL |
| Macronutrients | 12.5 mL |
| Micronutrients | 0.625 mL |
| Iron Source | 0.625 mL |
| Vitamins | 0.25 mL |
| Myo-Inositol | 25 mg |
| Sucrose | 7.5 g |
| Agar | 2 g |
| BAP (6-Benzylaminopurine) | 0.750 mL |
| IAA (Indole-3-Acetic Acid) | 0.125 mL |
Detailed Role of BAP and IAA in Plant Tissue Culture
1. BAP (6-Benzylaminopurine)
Type: Synthetic Cytokinin
Chemical Formula: C₁₂H₁₁N₅
Functions / Role:
- Promotes Cell Division (Cytokinesis):
BAP enhances mitotic activity, leading to rapid cell division and multiplication of plant cells in culture. - Shoot Induction and Proliferation:
It stimulates the initiation and growth of shoot buds from callus, explants, or nodal segments.
– Commonly used for shoot regeneration in tissue culture. - Delays Senescence:
BAP helps in retarding leaf yellowing and tissue aging by maintaining chlorophyll and protein levels. - Overcomes Apical Dominance:
It suppresses the dominance of the apical bud, allowing axillary buds to grow and form multiple shoots. - Enhances Chloroplast Development:
BAP supports chloroplast differentiation, improving photosynthetic potential in regenerated shoots. - Synergistic Role with Auxins:
When used with IAA or NAA, it helps in balancing shoot-to-root ratio, depending on the desired organogenesis.
2. IAA (Indole-3-Acetic Acid
Type: Auxin
Chemical Formula: C₁₀H₉NO₂
Functions / Role:
- Stimulates Cell Elongation:
IAA promotes cell enlargement and elongation, particularly in roots and stems, by increasing cell wall plasticity. - Root Initiation and Development:
It plays a key role in root formation, both in callus cultures and during shoot-to-root transition phases. - Promotes Vascular Differentiation:
IAA regulates the development of xylem and phloem tissues, improving nutrient and water transport. - Enhances Callus Formation:
In combination with cytokinins (like BAP), IAA helps in callus induction and subsequent organ differentiation. - Maintains Polarity in Tissue Culture:
Auxins like IAA guide directional growth (root downward, shoot upward) by maintaining morphogenetic polarity. - Regulates Gene Expression:
IAA influences genes responsible for cell division, elongation, and differentiation, ensuring balanced plant growth.
Plant Tissue Culture Of Banana Excel sheet:
10 Jan 2026
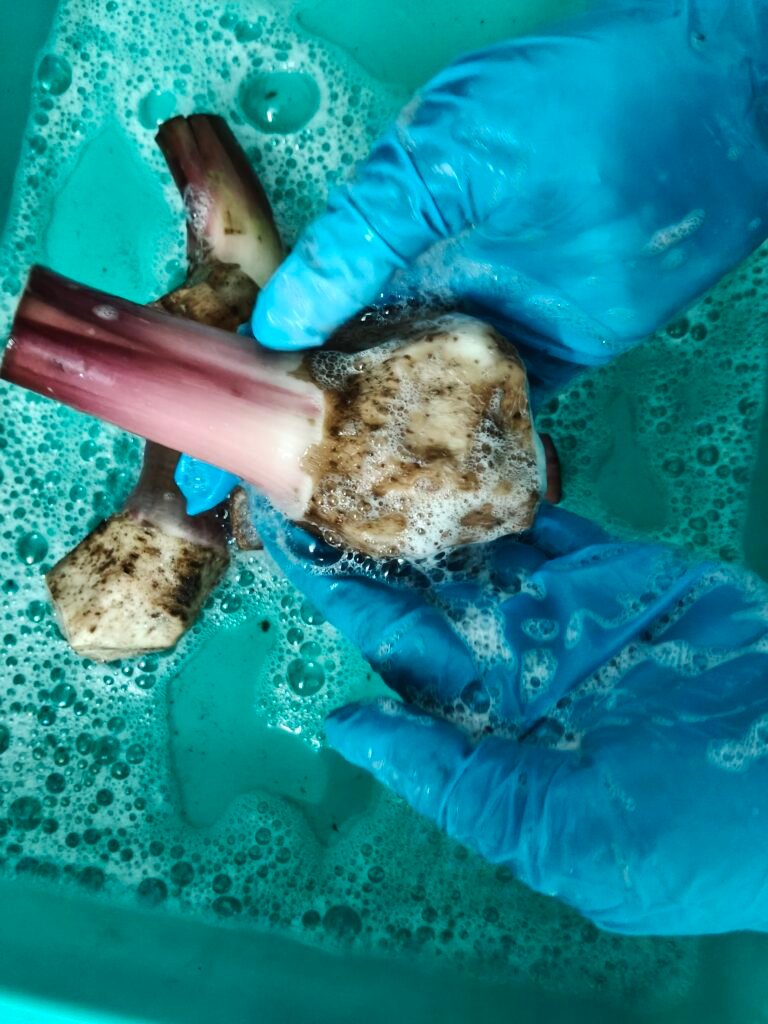
Today, G-9 (Grand Naine) banana mother plants were collected from the farm of Mr. Shankar Naikare’s located at Saygaon near Chas village, for use in plant tissue culture .


11 Jan 2026 – 14 Jan 2026
Banana explants were taken and cut into small pieces. They were cleaned properly, soaked in medicine to prevent infection, and then placed on MS medium. Plant tissue culture was done using 20 banana mother plants.





19-23 Jan 2026
I learned from Amisha how to shift banana plants at the second stage.

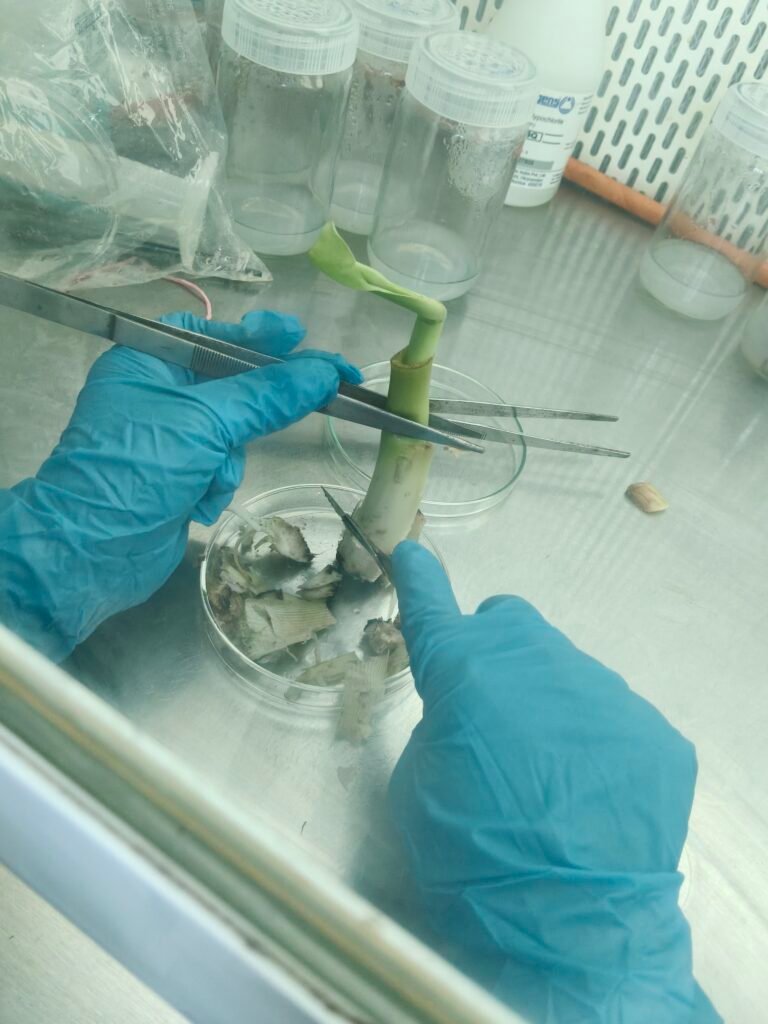

SUBCULTURING :-
2nd Stage :
After 30 days we prepared hormones media ( ms media + BAP + IAA )
After preparing the hormone-supplemented medium, the explant is taken from the culture bottle and cut into two parts on sterile paper. Then, each part is inoculate into fresh culture bottles containing the same hormone medium.
5 banana mother plants were shifted to the second stage. A total of 10 banana plants are now in the second stage.
Observation


After shifting to the stage -2, the banana plants showed good growth and no contamination was observed.
30 Jan 2026
- Prepared macro and micro nutrient stock solutions, and iron source solution.
- Learned the preparation of charcoal media from Amisha, which will be used in Stage 8 of the Banana Tissue Culture process.


