INTRODUCTION:
Fertigation is a method of fertilizing plants through an irrigation system. It involves injecting fertilizers into the water stream, allowing for precise and efficient delivery of nutrients to the roots of the plants.
Fertigation offers several benefits, including:
- Increased nutrient uptake
- Improved water efficiency
- Reduced fertilizer waste
- Enhanced crop yields and quality
Commonly used in agriculture, horticulture, and greenhouse production, fertigation is an effective way to optimize plant nutrition and promote healthy growth.
EC (Electrical conductivity):
EC (Electrical Conductivity) measures the concentration of dissolved salts in water. Reducing EC in water is important for several reasons:
- Plant growth: High EC levels can be toxic to plants, inhibiting growth and causing damage.
- Nutrient availability: Excessive salts can reduce nutrient availability, making it harder for plants to absorb essential minerals.
- Soil structure: High EC water can alter soil structure, reducing its water-holding capacity and affecting soil fertility.
- Irrigation system maintenance: High EC water can lead to clogged irrigation systems, pipes, and emitters due to mineral buildup.
- Crop quality: Excessive salt levels can affect crop quality, taste, and texture.
Reducing EC in water helps maintain optimal growing conditions, ensures plant health, and prevents potential problems in irrigation systems.
Objectives:
- Reduce EC in water.
- Manage pH levels.
- Improve water quality.
- Optimize fertigation process.
26/03/2025
Pranit and I visited the fertigation unit. Pranit explained how the unit works and showed me around. After that, we cleaned the area around the unit to make it safe and tidy.

27/03/2025
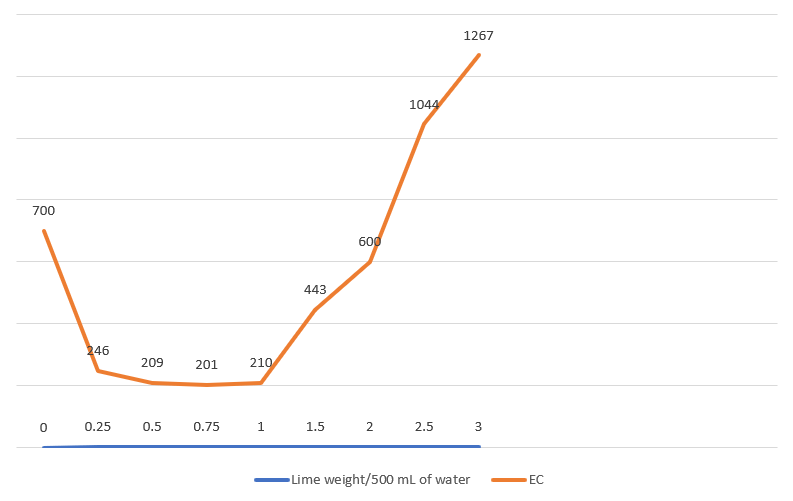
In order to reduce the Electrical Conductivity (EC) of the water, we initiated an experiment by collecting eight 500ml water samples. To each sample, we added varying weights of lime, ranging from 0.25 grams to 3 grams, in incremental steps. After carefully adding the lime to each sample, we thoroughly stirred the solutions to ensure uniform distribution. Next, we measured the EC of each sample to determine the effect of the lime addition on the water’s conductivity. Additionally, we collected 50ml subsamples from each of the original samples and proceeded to measure the pH levels, providing us with a comprehensive understanding of how the lime treatment impacted both the EC and pH of the water.
OBSERVATION:
| Lime weight (gm)/500 ml of water | EC | pH |
| 0 | 700 | 8.2 |
| 0.25 | 246 | 8.63 |
| 0.50 | 209 | 8.94 |
| 0.75 | 201 | 9.35 |
| 1.00 | 210 | 9.81 |
| 1.50 | 443 | 10.23 |
| 2.00 | 600 | 10.95 |
| 2.50 | 1044 | 11.26 |
| 3.00 | 1267 | 11.46 |

29/03/2025
We had a meeting with Dixit Sir, who reviewed our progress and informed us that our readings were satisfactory. He advised us to proceed with the next step, which involved working with a larger quantity of water. We collected 15 liters of water and conducted tests using three different weights of lime: 0.25 grams(0.25*15000/500=7.5), 0.5 grams(0.5*15000/500=15), and 0.75 grams(0.75*15000/500=22.50).
31/03/2025
Following our discussion in the meeting, we conducted the tests as planned and recorded our observations.
Observation:
| Lime weight(gm)/15 lit water | EC | pH |
| 22.50 | 467 | 9.44 |
| 15 | 429 | 9.24 |
| 7.5 | 550 | 8.97 |

We then presented our observations to Dixit Sir, who reviewed them and expressed his satisfaction with the results. He instructed us to proceed with the next step, which involved balancing the pH levels using Oxalic acid.
01/03/2025
To balance out the pH, we prepared a 10-liter water sample, to which we added 10 grams of lime, calculated based on the formula (0.50 x 10,000) / 500. The initial EC of the water was 361, and the pH before adding Oxalic acid was 9.20. Next, we withdrew a 1-liter subsample from the main 10-liter sample and began titrating it with Oxalic acid. We started with an initial dose of 0.09 grams and incrementally increased it to 0.15 grams, carefully monitoring the pH adjustments to achieve the desired balance. The pH should be between 6.5-7.
Observations:
| Weight of Oxalic Acid(gm) | pH |
| 0 | 9.20 |
| 0.09 | 7.28 |
| 0.10 | 7.18 |
| 0.11 | 6.67 |
| 0.15 | 6.10 |

02/04/2025
We repeated the experiment with 10 liters of water, but this time we added only 5 grams of lime. We observed and recorded the changes, noting differences in pH and other properties. This helped us understand how the amount of lime affects the solution.
OBSERVATION:
EC=572
| Weight of Oxalic Acid(gm) | pH |
| 0 | 8.55 |
| 0.09 | 7.88 |
| 0.10 | 7.25 |
| 0.11 | 6.76 |
| 0.15 | 6.26 |
05/04/2025
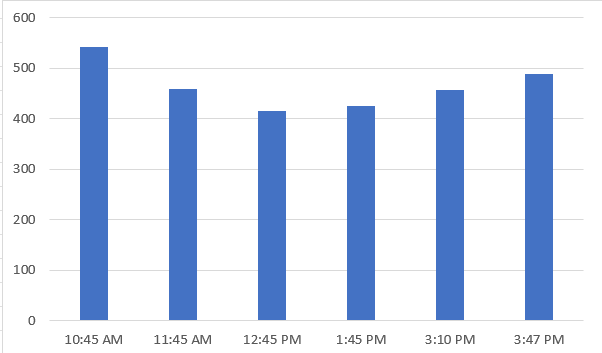
We observed that the electrical conductivity (EC) of the water with added lime decreased over time. To investigate this further, we prepared two new samples of 500 ml water each. In one sample, we added 0.50 grams of lime, and in the other, we added 0.25 grams of lime. We then measured the EC of both samples over a period of 1 hour to see how the lime affected the water’s conductivity.
OBSERVATIONS:
For 0.25 gm of Lime:
| Time | EC |
| 10:45 AM | 543 |
| 11.45 AM | 459 |
| 12:45 PM | 415 |
| 01:45 PM | 426 |
| 02:45 PM | 458 |
| 03:47 PM | 488 |
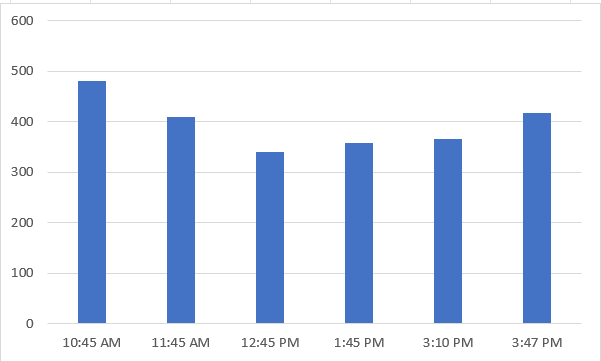
For 0.50 gm of Lime:
| Time | EC |
| 10:45 AM | 481 |
| 11:45 AM | 410 |
| 12:45 PM | 341 |
| 01:45 PM | 358 |
| 02:45 PM | 366 |
| 03:47 PM | 417 |

We prepared a new solution by mixing 5 liters of water with 5 grams of lime. Our goal was to measure the electrical conductivity (EC) of this solution. We then added oxalic acid to the lime-water mixture and observed the changes in EC.
OBSERVATION:
| Weight of Oxalic Acid(gm) | pH | EC |
| 0 | 9.38 | 483 |
| 0.09 | 7.13 | 429 |
| 0.10 | 7.00 | 426 |
| 0.11 | 6.88 | 422 |
| 0.15 | 6.75 | 414 |

06/04/2025
We had an weekly review meeting, where we discussed the progress of ongoing projects and addressed any challenges or concerns.
After the weekly meeting concluded, I received a water sample from Dixit sir for analysis. My task was to measure the EC and TDS of the sample.
Result:
EC=1830
TDS=915
07/04/2025
I shared my previous test results with Dixit sir, and he pointed out that lime dissolves very slowly, making hand stirring ineffective. He recommended switching to a magnetic or mechanical stirrer for better results. Since the mechanical stirrer wasn’t available in the lab, I decided to use a magnetic stirrer. I set the RPM to 600-750 and used a timer to take readings every 3 minutes. I repeated the process for few times, but unfortunately, the magnetic stirrer stopped working. Despite trying to troubleshoot the issue, I couldn’t get it to work again , which brought my experiment to halt.
Observations:
| Time of stirring | EC after Stirring |
| 0 min | 529 |
| 3 min | 479 |
| 6 min | 455 |
| 9 min | 438 |
| 12 min | 463 |

11/04/2025
I conducted an experiment using 5 samples of RO water with varying amounts of lime (0.05 to 0.25) and measured the Electrical Conductivity (EC) and pH levels.
Observations:
| Weight of lime (gm)/500 mL of water | EC | pH |
| 0 | 33 | 7.62 |
| 0.05 | 77 | 10.72 |
| 0.10 | 145 | 11.06 |
| 0.15 | 180 | 11.22 |
| 0.20 | 274 | 11.63 |
| 0.25 | 308 | 11.70 |
However, when I presented the results to Dixit Sir, I discovered that I had misheard his instructions. Instead of using RO water, we were supposed to use RO rejected water. To rectify this, Dixit Sir instructed me to obtain both RO rejected water and feed water (the water used for RO treatment). I will now proceed with the corrected experiment using the new water samples to ensure accurate and relevant results.

12/04/2025
Due to unforeseen circumstances, I was unable to obtain RO rejected water, so I proceeded with the water sample provided by Dixit Sir. I started by taking 500 ml of the water sample and measuring its initial Electrical Conductivity (EC) and pH levels. Next, I added lime to the water sample in incremental amounts, starting from 2 gm and going up to 6 gm. Interestingly, I observed that the EC initially dropped when I added 2 gm of lime, but as I continued to add more lime, the EC started to increase.
Observations:
| Weight of Lime(gm) | EC | pH |
| 0 | 1965 | 8.12 |
| 2 | 1696 | 9.94 |
| 4 | 2342 | 11.59 |
| 6 | 5250 | 12.03 |
Drawing from my previous experience with an oxalic acid test, where I noticed that adding oxalic acid not only lowered the pH but also reduced the EC, I decided to add oxalic acid to the water sample in incremental amounts ranging from 0.10 gm to 1.30 gm. I carefully recorded the observations after each addition, aiming to understand the effects of both lime and oxalic acid on the water’s EC and pH levels.
Observations:
| Weight of Oxalic Acid(gm) | pH | EC |
| 0 | 12.06 | 4981 |
| 0.10 | 12.02 | 4799 |
| 0.20 | 11.96 | 4587 |
| 0.30 | 11.86 | 4096 |
| 0.40 | 11.72 | 3484 |
| 0.50 | 11.49 | 2249 |
| 0.60 | 11.08 | 1880 |
| 0.70 | 9.90 | 1693 |
| 0.80 | 9.25 | 1701 |
| 0.90 | 8.26 | 1698 |
| 1.00 | 7.49 | 1732 |
| 1.10 | 7.00 | 1741 |
| 1.20 | 6.52 | 1741 |
| 1.30 | 6.46 | 1741 |
13/04/2025
I had planned to show my readings to Dixit Sir and proceed further, but unfortunately, I couldn’t meet him as he was attending to some guests. I decided to reattempt the test and check if I could reduce the Electrical Conductivity (EC) as desired. Additionally, I bought RO rejected water and the feed water as instructed by Dixit Sir.

14/04/2025
I conducted an experiment using 500 ml samples of feed water and RO rejected water. For each sample, I added lime in incremental amounts – ranging from 0.25 to 2 gm for feed water and 0.25 to 3 gm for RO rejected water. After each addition, I stirred the mixture for 2 minutes, using a timer on my phone, and then recorded the pH and Electrical Conductivity (EC) readings.
Observations of Feed Water:
| Weight of Lime(gm) | EC | pH |
| 0 | 793 | 7.93 |
| 0.25 | 805 | 8.97 |
| 0.50 | 688 | 8.53 |
| 0.75 | 646 | 9.32 |
| 1.00 | 594 | 10.24 |
| 1.25 | 714 | 10.80 |
| 1.50 | 879 | 11.12 |
| 1.75 | 1122 | 11.34 |
| 2.00 | 1540 | 11.56 |
Observations of RO Rejected Water:
| Weight of Lime(gm) | EC | pH |
| 0 | 1536 | 7.91 |
| 0.25 | 1552 | 8.23 |
| 0.50 | 1493 | 8.10 |
| 0.75 | 1384 | 7.93 |
| 1.00 | 1275 | 8.48 |
| 1.25 | 1194 | 8.16 |
| 1.50 | 1098 | 8.51 |
| 1.75 | 1047 | 9.14 |
| 2.00 | 979 | 9.59 |
| 2.25 | 1020 | 10.04 |
| 2.50 | 1060 | 10.53 |
| 2.75 | 1065 | 10.61 |
| 3.00 | 1136 | 11.00 |
Next, I took another 500 ml sample of RO rejected water, added 2 gm of lime, and then incrementally added oxalic acid to balance the pH, noting down all the readings throughout the process.
Observations:
| Weight of Oxalic Acid(gm) | pH | EC |
| 0 | 8.94 | 973 |
| 0.05 | 8.49 | 962 |
| 0.10 | 7.13 | 953 |
| 0.15 | 6.53 | 899 |
After presenting the results to Dixit Sir, he expressed satisfaction with the findings. Moving forward, our next step is to collect water samples from Mr. Shubham’s borewell. We will then conduct lime testing on these samples to assess their quality and determine the optimal lime dosage for treatment.

18/04/2025
As suggested by Dixit sir, we procured 20 liters of water from Mr. Shubham’s borewell. This water will be used for our specific needs or experiments.
25/04/2025
We did a lime test on 1 liter of water from Mr. Shubham’s borewell.
Observations:
| Weight of Lime | EC | pH |
| 0 | 1964 | 7.74 |
| 0.50 | 1964 | 8.48 |
| 1.00 | 2020 | 8.76 |
| 1.50 | 1986 | 8.71 |
| 2.00 | 1869 | 9.01 |
| 2.50 | 1793 | 9.33 |
| 3.00 | 1793 | 9.61 |
| 3.50 | 1821 | 9.89 |
| 4.00 | 1821 | 10.24 |
The EC drop when added 2.50 gm of lime.
Then I did test of Oxalic Acid.
| Weight of oxalic acid | EC | pH |
| 0 | 1793 | 9.33 |
| 0.05 | 1755 | 8.92 |
| 0.10 | 1755 | 8.35 |
| 0.15 | 1755 | 7.85 |
| 0.20 | 1720 | 7.41 |
| 0.25 | 1720 | 7.23 |
| 0.30 | 1755 | 7.21 |
| 0.35 | 1755 | 7.05 |
| 0.40 | 1792 | 7.00 |
We found that adding 2.50 grams of lime effectively reduced the water’s electrical conductivity (EC). Additionally, we discovered that adding 0.25 grams of oxalic acid helped balance out the pH level, achieving the desired water quality.
For 15 lit of water:
Weight of lime= (2.50 * 15000)/1000
=37.5 gm
Weight of Oxalic acid= (0.25 * 15000)/1000
=3.75 gm
Result:
Before Addition of Lime
EC= 1964
pH=7.74
After addition of lime
EC=1699
pH=12.04
After addition of Oxalic acid
EC=1680
pH=7.22
I presented the results to Dixit sir, and he reviewed them carefully. Based on the findings, he suggested that I proceed with determining the temporary hardness and permanent hardness of the same water sample. This additional analysis will provide more insight into the water’s properties and help us better understand its characteristics.
28/04/2025
I studied the procedure for determining the hardness of water and checked the laboratory inventory to ensure that all required chemicals were available. However, upon reviewing the list, I found that some essential chemicals were missing from the lab. As a result, I’ll need to order them, which will cause a temporary delay in my work. Until the chemicals arrive, I’ll have to put my project on hold, waiting for the necessary materials to proceed with the experiment.
30/04/2025
To determine the temporary hardness of water, I employed the boiling method. I started by taking a 100 ml water sample, which I then boiled on high flame for 2 minutes. After boiling, I let the sample cool down. Upon measuring the volume after cooling, I noticed some water had evaporated. To restore the original volume, I added distilled water to bring it back up to 100 ml. To calculate the temporary hardness, I measured the electrical conductivity (EC) of the water sample before and after boiling.
Result:
EC before boiling = 1768
EC after boiling = 1652
Temporary hardness = EC before boiling – EC after boiling
=1768-1652
= 116
02/05/2025
After analyzing the temporary hardness of the water sample using the boiling method, I presented the results to Dixit sir. Based on the findings, he observed that the temporary hardness was relatively low, while the permanent hardness was significantly high. Moving forward, our next objective is to determine the concentrations of sulphate and chloride ions in the water sample. To achieve this, we will employ specific chemical tests. For sulphate analysis, we will use barium chloride, which reacts with sulphate ions to form a precipitate, allowing us to quantify the sulphate content. Similarly, for chloride analysis, we will use silver nitrate, which reacts with chloride ions to form a precipitate, enabling us to calculate the chloride concentration.
03/05/2025
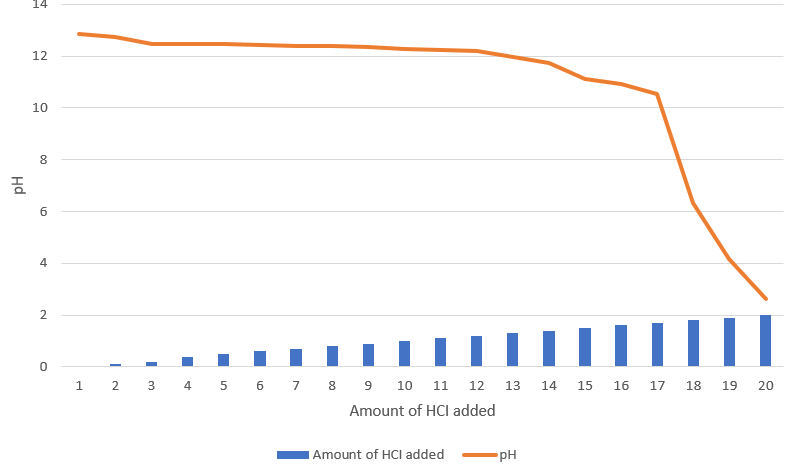
Due to the unavailability of barium chloride in the lab, I had to prepare it from barium hydroxide.
Procedure:
- Take 1.72 gm of Barium Hydroxide.
- Dissolve in 60 mL of Distilled water.
- Slowly add HCI ( Hydrochloric acid) , pH should become neutral (7).
- Dilute the solution with 40 mL of Distilled water.

Result:
| Amount of HCI added | pH |
| 0 | 12.85 |
| 0.1 | 12.73 |
| 0.2 | 12.48 |
| 0.4 | 12.47 |
| 0.5 | 12.45 |
| 0.6 | 12.44 |
| 0.7 | 12.41 |
| 0.8 | 12.39 |
| 0.9 | 12.35 |
| 1.0 | 12.29 |
| 1.1 | 12.25 |
| 1.2 | 12.20 |
| 1.3 | 11.95 |
| 1.4 | 11.74 |
| 1.5 | 11.12 |
| 1.6 | 10.92 |
| 1.7 | 10.55 |
| 1.8 | 6.33 |
| 1.9 | 4.15 |
| 2.0 | 2.64 |
05/05/2025
Hydrochloric acid (HCl) is a gas that’s often stored in liquid form. When the bottle is opened, some gas escapes, changing the acid’s concentration. To determine the exact concentration of HCl, I first needed to standardize the sodium hydroxide (NaOH) solution I would use for titration. To do this, I used potassium hydrogen phthalate (KHP), a known standard, to titrate the NaOH and determine its precise concentration. Once I knew the NaOH concentration, I could then use it to titrate the HCl sample and calculate its exact concentration.
Since liquid sodium hydroxide (NaOH) wasn’t available in the lab, I prepared the solution myself using NaOH pellets.
Procedure:
- Take 50 mL distilled water in a beaker.
- Slowly add 4 gm of NaOH pellets in it.
- Stir the solution gently. The distilled water heats after addition of pellets.
- Let the solution cool down and then add 50 mL of Distilled water to make it up to 100 mL.
After preparing the NaOH solution from pellets, I followed the following procedure to determine the concentration of NaOH.
Procedure:
- Take 0.903 gm of Potassium Hydrogen phthalate (KHP).
- Dilute it with 50 mL distilled water.
- Add 2-3 drops of Phenothaline Indicator.
- Fill the burette with NaOH solution.
- Titrate the KHP solution slowly till the sample terns into pink color. Note down the burette reading.
Reference:https://youtu.be/J8gDdy6y_j8?si=xE6N-iRQG3CYcxws


Then I followed the following procedure to determine the HCL concentration:
Procedure:
- Fill the burette with NaOH solution.
- Take 10 mL HCL.
- Add 2-3 Drops pf Phenophthalein indicator.
- Titrate the HCL with NaOH and Note down the burette reading.
Reference:https://youtu.be/VADhF7M8bJ8?si=ET621y9CTVKqj5EU


06/05/2025
After reviewing my results, Dixit sir gave his approval, saying they were satisfactory. He then suggested moving forward with two new tasks. First, we would calculate the amount of sulphate ions present in the water sample. Second, we would prepare ammonium oxalate by reacting ammonia with oxalic acid. The purpose of preparing ammonium oxalate was to use it to detect calcium ions in the water. When ammonium oxalate reacts with calcium ions, it forms calcium oxalate, which precipitates out of the solution. This precipitation reaction would help us determine the presence and potentially the amount of calcium ions in the water sample.
I followed the following procedure to prepare ammonium oxalate from Ammonia and oxalic acid.
Procedure:
- Take 1.26 gm of Oxalic Acid in a beaker.
- Dilute the oxalic acid with 50 mL distilled water.
- Check the pH of solution.
- Add drop wise ammonia till the pH becomes 3.
Observations:
| Amount of Ammonia Added | pH |
| 0 | 1.42 |
| 0.2 | 1.49 |
| 0.4 | 1.52 |
| 0.6 | 1.53 |
| 0.8 | 1.54 |
| 1.0 | 1.59 |
| 1.2 | 1.77 |
| 1.4 | 1.86 |
| 1.6 | 1.92 |
| 1.8 | 2.12 |
| 2.0 | 2.32 |
| 2.2 | 2.49 |
| 2.4 | 3.03 |
07/05/2025
Now that I know the concentration of hydrochloric acid (HCl), I’m ready to prepare barium chloride. I started by dissolving 1.713 grams of barium hydroxide in 50 mL of distilled water. Then, I slowly added HCl dropwise to the solution while monitoring the pH. As I added the HCl, the pH gradually decreased until it dropped drastically, indicating the endpoint of the reaction. At this point, I stopped adding HCl, knowing that barium chloride had formed. To finalize the solution, I added another 50 mL of distilled water to make the total volume up to 100 mL.
Observations:
| Amount of HCL added | pH |
| 0 | 12.39 |
| 0.1 | 12.35 |
| 0.2 | 12.31 |
| 0.3 | 12.23 |
| 0.4 | 12.10 |
| 0.5 | 11.98 |
| 0.6 | 11.81 |
| 0.7 | 11.66 |
| 0.8 | 11.51 |
| 0.9 | 11.39 |
| 1.0 | 11.13 |
| 1.1 | 7.38 |
Then I followed the following procedure to determine the Mass of sulphate ion in the water.
Procedure:
- Take 100 mL of water sample in a beaker.
- Add 5 mL of HCL in water sample.
- Heat the sample to 80-90 degree Celsius in the oven for better precipitation.
- Add 10 mL of Barium Chloride in the solution slowly with constant stirring.
- Keep the solution on a hot water bath at 80-90 degree Celsius temperature for 60 minutes. This allows the fine BaSo4 particles to coagulate into filterable size.
- Weigh a clean filter paper.
- Filter the hot mixture through it using a funnel.
- Carefully transfer all precipitate onto the filter paper using a glass rod.
- Dry the filter paper in an oven at 105-110 degree Celsius for 1 hour.
- Cool the filter paper.
- Weigh the filter paper with precipitate.


09/05/2025
Stock solutions were prepared to determine the total and permanent hardness of water. The preparation procedure is outlined below:
1)EDTA solution:
- Weigh 73.06 gm of EDTA.
- Dilute EDTA with 300 mL of Distilled Water.
- Heat at 50 degree Celsius.
- Add NaOH pellets to balance the pH (pH should be around 8).
- If pH becomes more than 8 add HCL.
Reference: https://youtu.be/EO_W0SrgGQM?si=xJqS3nAwUrh9-Gt6

2)Ammonia Buffer:
- Weigh 16.9 gm of NH4CI.
- Dilute NH4CI in 150 mL Distilled Water.
- Mix the solution evenly.
- Add 14.5 mL of Ammonia solution.
- Transfer this solution in a conical flask and make the volume upto 250 mL with Distilled Water.
Reference:https://youtu.be/NI17LAGhaM4?si=5Bs2IAv590TqrVXB
3)EBT Indicator:
- Weight 0.4 gm of EBT.
- Dilute EBT with 100 mL NaCl solution.
Reference:https://youtube.com/shorts/u7q_tOvxRyE?si=U1IoI4xOoqxUPc2F
10/05/2025
Conducted a test to check the Total and permanent hardness of water.
1)Total Hardness:
- Take 20 mL of water sample.
- Add 5 mL of Ammonia Buffer.
- Add 2 drops of EBT.
- Titrate the solution against EDTA solution.
- Color should change from Wine red to steel blue.
Burette reading=0.5 mL
Calculation:
Total Hardness = (Volume of EDTA consumed * 1000) /Volume of Water Taken
=(0.5 * 1000) / 20
=25 ppm
2)Permanent Hardness:
- Take 100 mL water sample.
- Boil at 100 degree Celsius.
- Cool and filter.
- Take 20 mL from boiled water.
- Add 5 mL ammonia buffer and 2 drops of EBT indicator.
- Titrate against EDTA solution.
- Color should change from Wine red to steel blue.
Burette reading=0.3 mL
Calculation:
Permanent Hardness = (Volume of EDTA consumed * 1000) / Volume of water taken
=(0.3* 1000) / 20
=15 ppm
3)temporary Hardness:
Temporary Hardness = Total Hardness – Permanent Hardness
=25 – 15
=10 ppm
Reference:https://vadic.vigyanashram.blog/2022/03/28/hardness-of-water-2/

11/05/2025
Now that I’ve determined the amount of sulphate ions in the water, I can calculate the amount of calcium sulphate present. Calcium sulphate is a compound that forms when calcium ions combine with sulphate ions. By using the mass of sulphate ions I calculated earlier, I can figure out how much calcium sulphate is dissolved in the water.
Molar Mass of Sulphate = 136.14 g/mol
Molar mass of Calcium sulphate = 96.06 g/mol
Mass of Calcium Sulphate = Mass of Sulphate * (Molar mass of sulphate / Molar mass of Calcium Sulphate)
= 135.87 * ( 136.14/96.06)
= 192.93 mg/lit
12/05/2025
With the amount of calcium sulphate in the water determined, I can now calculate the amount of ammonium oxalate needed to precipitate out the calcium ions. Ammonium oxalate reacts with calcium ions to form calcium oxalate, which precipitates out of the solution. By knowing the amount of calcium ions present, I can determine the exact amount of ammonium oxalate required to effectively remove the calcium ions from the water through this precipitation reaction.
Calcium Sulphate concentration in water= 192.93 mg/lit
Now,
Calcium Sulphate in 500 mL of water = 192.93 mg/lit * 0.5 lit
= 96.40 mg
= 0.096 g
Convert to moles,
Molar Mass of CaSO4 = 136.14 g/mol
Therefore,
Moles= 0.096/ 136.14
=7.09 * 10^-4 mol
Reaction is 1:1.
Use of 0.5 M of Ammonium Oxalate
Volume needed = 7.09 * 10^-4 / 0.5
= 1.418 * 10^-3 lit
= 1.42 mL
For 500 mL of water sample based on the amount of Calcium sulphate we required approximately 1.5 mL of Ammonium oxalate.
Procedure to determine mass of Calcium Oxalate:
- Take 500 mL of water sample , heat at 50 degree Celsius for better precipitation.
- Add 1.5 mL of Ammonium Oxalate with continuous stirring.
- Keep the solution warm for 15-20 min.
- After 20 min white precipitation appears on water.
- Take weight of filter paper.
- Filter the solution through a funnel with filter paper.
- Wash the precipitation with distilled water to remove impurities.
- Keep the filter paper in oven at 105 degree Celsius for 2 hrs.
Calculation:
Weight of Filter paper ( W1 ) = 1.080 gm
Weight of filter paper + Precipitation ( W2 ) = 1.105
Mass of Calcium Oxalate = W2 – W1
= 1.105 – 1.080
= 0.025 g
= 25 mg

13/05/2025
Since I forgot to assess the impact on EC during the initial experiment, I had to repeat the entire procedure to significantly investigate the effects on EC.
Observation:
EC of water before addition of Ammonium oxalate = 2067
EC of water after addition of Ammonium oxalate = 2796
EC of water after removal of Precipitate = 1965
Therefore,
Reduction in EC = 2067- 1965
=102

14/05/2025
Now, that I want to increase the concentration of ammonium oxalate, I followed the following procedure to make the concentrated solution:
Ammonium oxalate of 1M
Molar mass of Oxalic acid = 126.07 g/mol
Preparation of 100 mL
Quantity of oxalic acid = 0.1 lit * 126.07 g/mol
= 12.60 g
Ammonia = 25% ~ 14.5 M
Required 0.2 mol of NH3
Therefore,
Quantity of NH3 = 0.2/14.5
= 13.8 mL
- Take 70 mL of Distilled water in a beaker.
- Add 12.60 gm of Oxalic Acid in it.
- Stir the solution till oxalic acid dissolves.
- Add drop wise 13.8 mL of Ammonia and check the pH, should near to 3.
- After mixing pour the solution in 100 mL flask and make the volume to mark with distilled water.

16/05/2024
Today, I had to determine the concentration of ammonia to check if it still matches the given concentration. If the concentration had changed, I would need to repeat the procedure based on the newly calculated concentration of ammonia.
Acid solution for Titration;
1 M oxalic acid
Procedure:
Molar mass of oxalic acid = 126.07 g/mol
For 100 mL
Quantity of oxalic acid = 0.1 lit * 126.07 g/mol
= 12.60 g
- Rinse the burette first with distilled water and then with prepared Oxalic acid solution.
- Fill the burette till mark with Oxalic acid solution.
- Take 10 mL sample of Ammonia in a beaker.
- Add 2-3 drops of Phenolphthalein Indicator.
- Titrate it with oxalic acid till the color changes from pink to colorless.
- Note down the burette reading.

Calculation:

17/05/2025
Since the concentration had changed, I now had to re-prepare the ammonium oxalate solution , following the same procedure but using a different quantity of ammonia.
Ammonia = 18% ~ 11.81M
Required 0.2 mol of NH3
Therefore,
Quantity of NH3 = 0.2/11.81
= 16.9 mL
- Take 70 mL of Distilled water in a beaker.
- Add 12.60 gm of Oxalic Acid in it.
- Stir the solution till oxalic acid dissolves.
- Add drop wise 16.9 mL of Ammonia and check the pH, should near to 3.
- After mixing pour the solution in 100 mL flask and make the volume to mark with distilled water.
18/05/2025
With the new calculations in hand, I had to re-determine the mass of calcium oxalate in the water sample. By doing so, I was able to obtain a precise measurement of the calcium oxalate mass which was essential for further analysis.
Calculation:

Procedure:
- Take 500 mL of water sample , heat at 50 degree Celsius for better precipitation.
- Add ~0.71mL of Ammonium Oxalate with continuous stirring.
- Keep the solution warm for 15-20 min.
- After 20 min white precipitation appears on water.
- Take weight of filter paper.
- Filter the solution through a funnel with filter paper.
- Wash the precipitation with distilled water to remove impurities.
- Keep the filter paper in oven at 105 degree Celsius for 2 hrs.

Result:
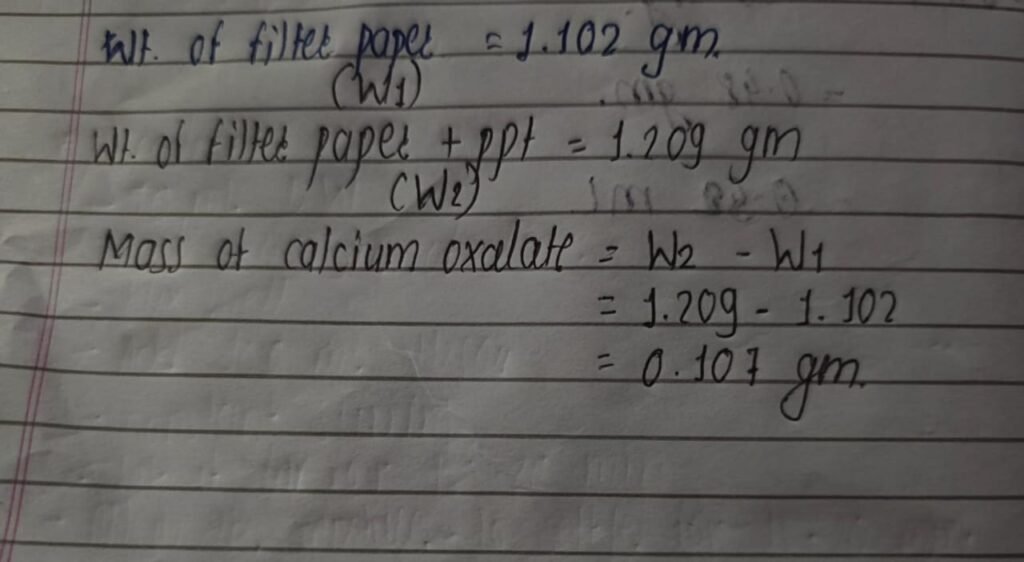
19/05/2025
I prepared Calcium Sulphate to precipitate Calcium Oxalate with the help of Ammonium Oxalate from Calcium Hydroxide (Ca(OH)2) and Sulphuric Acid (H2SO4). For this firstly I had to prepare dilution of Sulphuric Acid, for which I followed the following steps:
Preparing 100 mL of 1M H2SO4
H2SO4 is ~98% w/w
Density ~1.84 g/mL
Molarity = 18M
Dilution Formula :
M1V1 = M2V2
Where,
M1 = 18 M
V1 = Volume to find
M2 = 1M
V2 = 100 mL
Therefore,
V1 = (M2 * V2) / V1
= (1 * 100)/18
= 5.56 mL
Procedure:
- Take 5.56 mL of Sulphuric Acid in a Beaker
- Add drop by drop 70 mL of distilled water.
- Let the solution cooled down.
- Make the volume up to 100mL with distilled water.
After preparing the diluted Sulphuric Acid, I then calculated the mass of calcium hydroxide required to prepare calcium sulphate. This calculations was essential to ensure that I had the correct amount of Calcium hydroxide to react with the sulphuric acid and produce the desired amount of calcium sulphate.

I then followed the following procedure to prepare calcium sulphate, carefully reacting the calcium hydroxide with the diluted sulphuric acid:
- Take 50 mL of distilled water in a beaker.
- Add 7.40 gm of Calcium Hydroxide and dissolved well.
- Then add 100 mL of diluted H2SO4 in the solution slowly with constant stirring.
- Let it stir for 20 min on magnetic stirrer.
- Allow the mixture to settle for 15-20 min.
- After that filter the solution from a filter paper through a funnel.
- Dry the filtering in an oven for 2 hours at 120 degree celcius.
20/05/2025
Now that I have the Calcium sulphate , I can proceed to precipitate calcium oxalate using ammonium oxalate and calcium sulphate. For this, I need to calculate the amount of both chemicals required for a 100 mL water sample.

21/05/2025 & 23/05/2205
As there were a few problems with the oven in the lab, and I needed to use the oven, I therefore had to put my experiment on hold.
24/05/2025
I followed the following procedure to precipitate calcium oxalate from the water sample:
- Take 500 mL of water sample in a beaker.
- Add 2 gm of Calcium sulphate in the water.
- Stir the solution for 30-40 min on a magnetic stirrer.
- Filter the solution to remove undissolved CaSO4.
- Then add 7.5 mL of 1M ammonium oxalate with constant sirring.
- Let the solution for some time till the precipitation appears.
- Weigh the filter paper.
- Filter the solution through filter paper.
- Dry the filter paper in oven for 1 hour at 62 degree celcius.
- After drying take the weight of filter paper with precipitate.
Calculations:
Mass of Calcium oxalate= W2-W1
where,
W1= Weight of filter paper
W2= Weight of filter paper with precipitate
Therefore,
Mass of Calcium oxalate = 1.298 – 1.111
= 0.187 gm
= 187 mg
Precipitation of Calcium Oxalate-
Molecular Weight of Calcium Sulphate = 136.14 g/mol
Molecular Weight of Ammonium Oxalate = 124.09 g/mol
% Precipitation = ((124.09 * 0.187)/136.14)× 100
= 17%
26/05/2024
As the precipitation rate was relatively low, we decided to adjust the ratio to 1:2. Consequently, I used double the amount of ammonium oxalate compared to the previous experiment. This modification aimed to enhance the precipitation reaction.
- Take 500 mL of water sample in a beaker.
- Add 2 gm of Calcium sulphate in the water.
- Stir the solution for 30-40 min on a magnetic stirrer.
- Filter the solution to remove undissolved CaSO4.
- Then add 15 mL of 1M ammonium oxalate with constant sirring.
- Let the solution for some time till the precipitation appears.
- Weigh the filter paper.
- Filter the solution through filter paper.
- Dry the filter paper in oven for 1 hour at 62 degree celcius.
- After drying take the weight of filter paper with precipitate.
Calculations:
Mass of Calcium oxalate= W2-W1
where,
W1= Weight of filter paper
W2= Weight of filter paper with precipitate
Therefore,
Mass of Calcium oxalate = 1.619 – 1.112
= 0.505 gm
= 505 mg
Precipitation of Calcium Oxalate-
Molecular Weight of Calcium Sulphate = 136.14 g/mol
Molecular Weight of Ammonium Oxalate = 124.09 g/mol
% Precipitation =((124.09 * 0.505)/136.14)× 100
= 46 %
28/05/2025
As the precipitation rate increased when the amount of ammonium oxalate was increased, I decided to further adjust the ratio from 1:2 to 1:3 to observe the effect on the precipitate. By increasing the proportion of ammonium oxalate, I aimed to determine whether this would lead to corresponding increase in the precipitation rate or if it would reach an optimal point.
- Take 500 mL of water sample in a beaker.
- Add 2 gm of Calcium sulphate in the water.
- Stir the solution for 30-40 min on a magnetic stirrer.
- Filter the solution to remove undissolved CaSO4.
- Then add 21.5 mL of 1M ammonium oxalate with constant sirring.
- Let the solution for some time till the precipitation appears.
- Weigh the filter paper.
- Filter the solution through filter paper.
- Dry the filter paper in oven for 1 hour at 62 degree celcius.
- After drying take the weight of filter paper with precipitate.
Calculations:
Mass of Calcium oxalate= W2-W1
where,
W1= Weight of filter paper
W2= Weight of filter paper with precipitate
Therefore,
Mass of Calcium oxalate = 1.823 – 1.111
= 0.711 gm
= 711 mg
Precipitation of Calcium Oxalate-
Molecular Weight of Calcium Sulphate = 136.14 g/mol
Molecular Weight of Ammonium Oxalate = 124.09 g/mol
% Precipitation = ((124.09 * 0.711)/136.14)× 100
= 64.81%
30/05/2025
I showed the results to Dixit sir, and the results were satisfactory. Now, my next task is to analyze the cost to check whether this treatment is beneficial for farmers in larger quantities or not.
31/05/2025
To determine the feasibility for farmers, it is essential to scale the quantities proportionally and assess the economic and logistical practicality for treating 1000 liters of irrigation water.
SCALING CALCULATION FOR 1000 LIT WATER:
Scaling Factor =1000/0.5
= 2000
Scaled Reagent
| Reagent / Material | For 500 mL | For 1000 lit |
| 1M ammonium Oxalate | 22.5 mL | 45,000 mL (45 lit or 5.59 kg) |
03/06/2025
COST OF AMMONIUM OXALATE:
| Reagent / Material | Quantity | Unit price | Cost (INR) |
| Ammonium Oxalate | 5.59 kg | Rs.115/kg | Rs.643 |
COST OF AMMONIUM OXALATE PREPARATION:
| Reagent/Material | Quantity | Unit price | Cost (INR) |
| Ammonia | ~ 7 kg | Rs.22/kg | Rs. 154 |
| Oxalic Acid | ~ 5.7 kg | Rs.60/kg | Rs.342 |
| Total= 496/- |
If we purchase direct ammonium oxalate from the market, the cost is relatively high compared to preparing ammonium oxalate with the help of ammonia and oxalic acid.
AMOUNT OF AMMONIUM SULPHATE AS FERTILIZER:
Ammonium Sulphate = (1 /500)× 1,000,000 mL
= 2000 gm
= 2 kg
COST OF AMMONIUM SULPHATE:
| Reagent/Material | Quantity | Unit price | Cost (INR) |
| Ammonium Sulphate | 2 kg | Rs.200/kg | Rs.400 |
COST ANALYSIS:
Total Cost = 496 – 400
=96 Rs.
Link of Note prepared on costing:
https://drive.google.com/file/d/155Hn0chkefdRwhbOALlfSeYLjd011Div/view?usp=sharing




