1.INTRODUCTION
Plant tissue culture is a technique used to grows plant cells, tissues, or organs in a controlled laboratory .It involves taking small sample of plant tissue (called explant ) and placing them in a nutrient-rich medium ,where they can grow and develop into new plants. The technique takes advantage of plant cells totipotency ,which means that any cell from any area of the plant can be used to make brand new plant. Although tissue culture has been around since the beginning of the 18th century, plant tissue culture only began developing in 1898. Gottlieb Haberlandt , a German Botanist, made the first attempt to use the in vitro method.
2.ADVANTAGES
Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including:
The production of exact copies of plants that produce particularly desirable traits.
To quickly produce mature plants.
To produce a large number of plants in a reduced space.
The production of multiples of plants in the absence of seeds or necessary pollinators.
The regeneration of whole plants from plant cells that have been genetically modified.
The production of plants in sterile containers allows them to be moved with greatly reduced chances of transmitting diseases, pests, and pathogens.
To clean particular plants of viral and other infections and to quickly multiply these plants as ‘cleaned stock’ for horticulture and agriculture.
Storage of genetic plant material to safeguard native plant species
Stages of plant tissue culture:
- Initiation:
Explants (small plant samples) are taken from a donor plant and sterilized to remove contaminants. - Establishment:
Explants are placed in a nutrient medium, and the culture is established in a controlled environment. - Multiplication:
Cells or tissues grow and multiply in the medium, forming a callus or suspension culture. - Differentiation:
Cells or tissues differentiate into specific organs or tissues, such as roots, shoots, or leaves. - Shoot regeneration:
Shoots and roots develop from the differentiated cells or tissues. - Rooting:
Roots develop from the shoots, forming a complete plantlet. - Acclimatization:
Plantlets are gradually adapted to external conditions, such as light, temperature, and humidity. - Transplantation:
Plantlets are transferred to soil or other growing media, marking the final stage of tissue culture.
2.OBJECTIVES
To reduce the contamination rate in the plant tissue culture laboratory from 100% to 50%.
3.MATERIALS AND METHODS
MATERIALS:
| Glassware | Instruments |
| Conical flask | weighing balance |
| Test tubes | Magnetic stirrer |
| Petri plates | pH meter |
| Beaker | Laminar air flow |
| Culture bottles | Autoclave |
| Measuring cylinder | Hot air oven |
| Water distillation unit | |
| Refrigerator |
CHEMICALS:
| for MS Media | For Sterilization |
| Macronutrients | Ethanol |
| Micronutrient | Sodium hypochlorite |
| Agar Powder | Tween 20 |
| Vitamins | Bavistin |
| Growth regulators | Liquid soap |
| Amino acid | Dettol |
| HCl | Mercuric chloride |
| NaoH | |
| Sucrose | |
| Iron source |
OTHER MATERIALS:
| Test tube stand | Sterile autoclave water |
| forecep | tissue paper |
| Spatula | filter paper |
| Scalpel | cotton plug |
| Sterile surgical blade | scissors |
| Distilled water | autoclave bags |
METHODOLOGY
Sterilization
Sterlize the glassware such as culture bottle, measuring cylinder, flasks ,petri plates and beakers also sterilize other materials like blade holder, forecep, .Before sterilization, ensure that glassware is:- Clean and free of debris- Dried and free of water spots- Assembled and wrapped (if necessary).
Glassware sterilization is a crucial step in plant tissue culture to prevent contamination and ensure the success of the culture. Here are the common methods used for glassware sterilization:
1.Autoclaving: Expose glassware to high-pressure steam (121°C, 15 psi) for 15-30 minutes to kill microorganisms.
2. Dry Heat Sterilization: Place glassware in a hot air oven (160-180°C) for 1-2 hours to sterilize.
3. UV Radiation: Expose glassware to UV light (254 nm) for 30 minutes to 1 hour to sterilize.
After sterilization, glassware should be:- Removed from the sterilizer with sterile gloves or instruments
Placed in a sterile environment (e.g., laminar air flow)
Used immediately or stored in a sterile container for later use.
4 .PREPARATION OF STOCK SOLUTIONS:
1.Macroelement or Macronutrient stock (10X) or (20X)
| Macro elements | Stock volume 500ml | |
| Inorganic salts | Organic concentration as per table | Amount of salt after multiplying original concentration by 10 times or 20 times |
| NH4NO3 | 1650 mg/l | 16.5 g |
| KNO3 | 1900 mg/l | 19.0 g |
| KH2PO4 | 170 mg/l | 1.7 g |
| MgSo4.7H2O | 370 mg/l | 3.7 g |
| CaCl2.2H2O (MW 147 g) | 440 | 4.4 g OR |
| CaCl2 (fused) (MW 110.98) | 440 | 3.3 g |
For making 10X stock ,take 500ml distilled water in a 1 liter beaker ,weigh each salt ,add(one by one) and keep on dissolving each salt sequentially in a descending order as listed above (dissolve by magnetic stirrer or manual swirl),and finally make up the volume to 1000 ml by distilled water.
For making 20X stock ,take 250ml distilled water in a 1 liter beaker ,weigh each salt ,add(one by one) and keep on dissolving each salt sequentially in a descending order as listed above (dissolve by magnetic stirrer or manual swirl),and finally make up the volume to 500 ml by distilled water.
2.Microelement or Micronutrient stock (400X)
| Micro elements stock (400X) | Stock volume 250 ml | |
| Inorganic salts | Original concentration as per table | Amount of salt after multiplying original concentration by 100X and making final volume to 250 ml |
| H3BO3 | 6.2 mg/l | 620 mg |
| MnSO4.4H20 | 22.3 mg/l | 2230 mg |
| ZnSO4.7H2O | 8.6 mg/l | 860 mg |
| Na2MoO4.2H2O | 0.25 mg/l | 25 mg |
| CuSO4.5H2O | 0.025 mg/l | 2.5 mg |
| CoCl2.6H2O | 0.025 mg/l | 2.5 mg |
| KI | 0.83 mg/l | 83 mg |
For making 400X stock ,take 100 ml distilled water in a 250 ml beaker ,weigh each salt ,add (one by one) and keep on dissolving each salt sequentially in a descending order as listed above (dissolving by magnetic stirrer or manual swirl), and finally make up the volume to 250 ml by distilled water.
3. Vitamin stock (1000X)
| Vitamin stock (1000X) | Stock volume 50 ml | |
| Items | Original concentration as per table | Amount of item after multiplying 1000 times and making final volume to 50 ml |
| Thiamine HCl | 0.1 mg/l | 0.005 g |
| Pyridoxine HCl | 0.5 mg/l | 0,025 g |
| Nicotinic acid | 0.5 mg/l | 0,025 g |
| Glycine | 2.0 mg/l | 0.1 g |
For making 1000X stock, take 20 ml distilled water in a100 ml beaker, weigh each vitamin item,add (one by one) and keep on dissolving each vitamin sequentially in descending order as listed above (dissolve by magnetic stirrer or manual swirl), and make up the volume to 50 ml by distilled water.
4. Iron source stock (400X)
| Iron source stock (400X) | Stock volume 250 ml | |
| Items | Original concentration as per table | Amount of items after multiplying it with 400X and making final volume to 250 ml |
| FeSO4.7H2 O | 27.8 mg/l | 2.78 g |
| Na2EDTA.2H2O | 37.3 mg/l | 3.73 g |
For making 400X stock, take 70 ml distilled water in two 200 ml beaker ,weigh each item ,add in each separate beaker and dissolve each item. by continues stirring or gentle heating then mix both solution and finally make up the volume to 250 ml by distilled water.
5.Mesoinositol or Myoinositol -100 mg/l
Weigh 100 mg myoinositol for 1 litre MS media .If media volume is differ ,accordingly weighing of myoinositol will differ.
6.Sucrose-3%
Weigh 30 g of sucrose for 1 litre MS media .If media volume is differ ,accordingly weighing of sucrose will differ.
7.Cytokinin, Auxin stock preparation
BAP stock-100mg/100ml (which is 1mg/ml, 10mg/10ml, 100mg/100ml and 1000mg/1000ml) Weigh 100
mg of BAP, drop it in 250ml conical flask, dissolve it with ethanol or IM NaOH solution with few drops.
Make sure BA powder dissolve completely, and then make final volume to 100 ml with DW. Use this
stock for any working concentrations of MS media.
IBA stock-100mg/100ml (which is 1mg/ml, 10mg/10ml, 100mg/100ml and 1000mg/1000ml)
Weigh: 100 mg of IBA, in conical flask, dissolve it with ethanol or 1M NaOH solution with few drops . Make sure IBA
powder dissolve completely and then make final volume to 100 ml with distilled water.
Use the stock for any working concentrations of MS media.
8. Prepare working concentration from cytokinin, auxin stock
The formula is Stock concentration stock volume = working concentration Media volume
1000mg/1000ml x stock volume(ml) = 0.5 mg/1000ml × 500ml
stock volume= (0.5 x 500)/1000
stock volume = 0.25 ml
Therefore, pipette 250 µl and add into 500 ml media. It is important to make mathematical calculations
on plane paper before preparing the media.
9.pH level- Make pH of media to 5.8-5.9 by using 1M HCI or 1M NaOH
After finalizing pH ,make the final volume of media 1000ml (as per media requirement ).out of this ,pour media into beaker i.e 500 ml of media in 1000 ml beaker.
10. Agar
Weigh 8 g agar powder ,add in media swirl with glass rod then microwave the media for 14 min .Dissolve the agar completely .
After cooling the media for 5 min then pour the media in test tube .Each test tube carrying 15-20 ml media ,plug it with cotton plug or test tube cap .Bundle of 6-8 test tube with rubber band .Wrap it with autoclave bags and autoclave it for 20 min (121 degree Celcius ;15 psi pressure;20 min) .After Autoclaving the media keep it on safe platform.
Inoculate the media after 24 hrs.
Murashige and Skoog (MS) media preparation based on media volume
| Items | final volume | final volume | final volume | final volume | final volume |
| 1000 ml | 500ml | 250 ml | 100 ml | 750 ml | |
| DW | 500 ml | 25 ml | 100 ml | 50 ml | 400 ml |
| 10X Macro elements or 20X Macro elements | 100 ml 50 ml | 50 ml 25 ml | 25 ml 12.5 ml | 10 ml 5 ml | 75 ml 37.5 ml |
| 400X Microelements | 2.5 ml | 1.25 ml | 0.625 ml | 0.25 ml | 1.875 ml |
| 400X Iron source | 2.5 ml | 1.25 ml | 0.625 ml | 0.25 ml | 1.875 ml |
| 1000X Vitamin | 1 ml | 0.5 ml | 0.25 ml | 0.1 ml | 0.75 ml |
| Mesoinositol | 100 mg | 50 mg | 25 mg | 10 mg | 75 mg |
| Sucrose | 30 g | 15 g | 7.5 g | 3 g | 22.5 g |
| Agar | 8 g | 4 g | 2 g | 0.8 g | 6g |
SURFACE STERILIZATION OF EXPLANT:
STEP 1: Collect explant in distilled water jar in size of 1-2 inches .ie trim the explant to the desired size and shape.
STEP 2: Rinse the explant with tap water for 7-10 times.
STEP 3: Transfer the explant in 0.1% of bavistin solution for 1 min.
STEP 4:Repeat the rinse step 10-13 times with distiled water.
STEP 5:Rinse the explant thoroughly with liquid soap then rinse it with distilled water 5-6 times .
STEP 6:Rinse the explant thoroughly with Tween 20 .
STEP 7: Repeat the rinse step 8-10 times with distiled water.
STEP 8:Transfer the explant in 0.1% -0.4% of dettol solution for 2-3 min
STEP 9: Repeat the rinse step 3-5 times with distiled water.
STEP 10:Rinse the explant thoroughly with sterile water.
STEP 11: Transfer the explant in 1mg/ml HgCl solution for 3 min.This step carried out under laminar air flow.
STEP 12:Repeat the rinse step 3-5 times with sterile water.
INOCULATION OF CULTURE :
Inoculation of explant in explant culture refers to the process of introducing a small piece of tissue (explant) into a sterile growth medium, such as agar or broth, to initiate growth and development in plant tissue culture.
Inoculation of explant in culture under laminar air flow is a critical step in plant tissue culture. Laminar air flow provides a sterile environment, minimizing contamination risks. Here’s a step-by-step protocol:
Prepare the explant: Trim and sterilize the explant as described earlier.
- Turn on the UV light for 15-30 minutes before use. then switch on the blower .
- Wear sterile gloves, lab coat, and face mask .kept all material in UV light for 20 min.
- Switch On burner then Open the culture vessel
- Inoculate the explant
- Use sterile forceps or a scalpel to gently place the explant into the culture medium.
- Close the culture vessel
- wrap the edges with parafilm or tape.
- Label and date the culture vessel
- Use a marker to label the vessel with the explant name, date, and any other relevant information.
- Incubate the culture
- Place the culture vessel in a growth room or incubation chamber with suitable conditions (e.g., temperature, light, humidity).
- Monitor and subculture:
- Regularly check the culture for growth, contamination, and differentiation.
- Subculture the explant as needed to maintain healthy growth and prevent overcrowding.
- Ensure the cabinet is turned on and the air flow is laminar.
- Position the explant as desired (e.g., horizontal, vertical, or at an angle.
INCUBATION :
Placed the test tube in an incubation room with controlled conditions:
Temperature-25 degree celcius
Light – 18 hrs dark +6 hrs light
1.Preparation of stock solution.
August 2024
2.250 ml MS Media preparation.
3.Inoculation of explant (mulberry shoot).
4.Preparation of MS+BAP media .
5.Fumigation of tissue culture lab.
Result: Contamination occurs to the culture bottle and tubes;that was bacterial and fungal infection to we decide to perform fumigation in tissue culture lab.
On August 20, 2024, Harshad Sir visited our laboratory and demonstrated the best practices for media preparation. Under his guidance, we successfully prepared 500 ml of MS media, a defined medium for plant tissue culture. We learned the importance of precision and sterility in media preparation.
On August 23, 2024, Harshad Sir visited our laboratory and demonstrated the essential practices of surface sterilization and inoculation of explants. Specifically, he showed us how to sterilize and inoculate Napier grass explants, a crucial step in plant tissue culture. We learned about the importance of surface sterilization in removing microbial contaminants and the precise techniques for inoculating explants to initiate culture.
Result:Unfortunately, within the 5-day incubation period, bacterial contamination occurred in our Napier grass explant cultures.
Following Harshad Sir’s instructions, I prepared MS liquid media without agar and inoculated the Napier grass explant. I carefully executed the protocol, ensuring that the explant was properly sterilized and then transferred to the liquid media. The explant was then incubated.Unfortunately, out of the 7 culture bottles I prepared, 4 showed contamination within 6 days.
Conclusion :Observations revealed that the autoclave’s gasket was faulty, leading to a loss of pressure and compromising the sterilization of our media, which is a critical step in our experiment.

September 2024
In the first week of September, I prepared 20 jars with varying concentrations of auxin and cytokinin, and also prepared 20 test tubes containing explant Moringa seeds for tissue culture experimentation.
I Visited to SPPU University Department of Botany to observe and learn about tissue culture practices . Interacted with faculty and researchers to gain a deeper understanding of their work.- Explored the facilities and equipment used for tissue culture in the department.
I prepared 50 bottles and 3 jars of explant mulberry shoots and moringa shoots, from which 60% got contaminated within 7 days.
In this preparation, we adopted a modified bottle sterilization procedure, similar to SPPU University’s protocol, where we wrapped the test tubes with parafilm prior to autoclaving, and only opened them inside the autoclave during the inoculation process.
On 15 September, the cleaning procedure for Laminar Air Flow and AC filters was discussed by Dixit sir, Prasad sir, and Mahesh sir.
Additionally, Dixit sir told me the solution for removing black mold that had grown on the wall surface. He advised me to clean the entire surface of the lab walls where the fungi had grown, using a 5% sodium hypochloride solution.
:Aaman cleaned the laminar air flow by removing the filter, vacuuming it, and then spraying a 5% sodium hypochloride solution.we subsequently wiped the filter with 70% ethanol, let it dry overnight, and then reinstalled it in the laminar. Following this, we performed fumigation in the laboratory.
In the last week of September, I prepared 50 jars containing Moringa shoots as explants.
November 2024
In the last week of November, we visited the Vivekanand Institute of Biotechnology, located in Nimpith village, Kolkata. There, we learned tissue culture practices, from explant collection to primary hardening, and practiced techniques using banana suckers as explants.

learning inoculation of explant
SELECTION OF PLANT AND SUCKER CUTTING (VIDEO):
https://drive.google.com/file/d/1FS5V-vpAKG7AQNJzQrG0c7OF4XXKgHGZ/view?usp=drivesdk
EXPLANT CUTTING (VIDEO):
https://drive.google.com/file/d/1F8aa2W8595nZCv2zDNtCMSjSJTV7t5tv/view?usp=drivesdk
SUBCULTURING (VIDEO) :
https://drive.google.com/file/d/1FFsXIu3PMfBuLZJX49SRNJBOAVWKAGpN/view?usp=drivesdk
HARDENING (VIDEO) :
https://drive.google.com/file/d/1EUhdhHkZX1mNP9NJY9eJ9nMj_WRWZUQ7/view?usp=drivesdk
BANANA PLANT TISSUE CULTURE BOOK PDF
[ VIVEKANAND INSTITUTE OF TECHNOLOGY,NIMPITH ]
https://drive.google.com/file/d/1DRWHD9wPEl1uYUPG6rK3tjckjwvmzTao/view?usp=drivesdk
BANANA PTC ,VIGYAN ASHRAM (VIDEO)
https://drive.google.com/file/d/1E29vO6pDqhL0nzUK4qfCw992ZJveaggD/view?usp=drivesdk
December 2024
In the first week of December, we followed a glass bottle washing procedure.
The procedure for washing the jars is as follows:
- Autoclave the jars.
- Soak them overnight in tap water.
- Soak the jars in chromic acid (minimum 0.1%) for 30 minutes.
- Wash them with liquid soap in running tap water.
- Dry the jars.
- Inspect the bottles and caps under light to avoid contamination.
- Perform dry sterilization of the jars (not the caps) for 12 hours at 180°C.
- Finally, wipe the caps with ethanol and sterilize them under UV light in a laminar flow cabinet for 1 hour.
In the second week of December, we prepared stock solutions by followed by table no.4.1-4.4 .
We prepared MS basal media for initiation . We prepared 30 jars and also performed sterilization of the materials required for the initiation of explants in culture.

Selection of mother plant
- Select plants with no visible signs of disease or pests.
- Select banana varieties known for their high yields and desirable traits.
- Consider plant age: Select plants that are at least 6-12 months old.
- Keep detailed records of the plant’s origin, selection process, and laboratory testing results.
Collection and Washing Procedure
- Wash the suckers with tap water.
2.Cut it in desirable shape.
3.Place them in a dry tray, avoiding water.

4.Wash the suckers in running water for 15 minutes, ensuring they are under running tap water.

5.Cover them with a bandage or cloth to prevent material loss.
6Handle the plant material with sterilized gloves.
Soap Solution Washing
- Prepare a 1000ml soap solution by mixing 100ml liquid soap with 900ml double distilled water. [for 1 jar 30 ml of liquid soap with 270 ml of double distilled water].
- Wash the material with gloved hands for 5 minutes.
- Place the material in a glass beaker and remove the soap solution.

Cutting and Washing
- Cut the material to the proper size using a knife, removing the upper layer.
- Wash the material once before placing it in a jar.

Savlon Solution Soaking
- Prepare a Savlon solution by mixing 10ml Savlon with 1000ml double distilled water.[for 1 jar 3ml of Savlon with 300ml of double distilled water].
- Soak the material for 15 minutes.
- Wash the material four more times with double distilled water.

Laminar Airflow Procedure
- Prepare a 1% sodium hypochlorite solution and soak the explant for 15 minutes.[ for 1 jar 50 ml of sod.hypochlorite with 250 ml double distilled water].
- Slowly wash the explant four times with double-distilled water.
- Cut the explant to the required final size (2-3 inches) on sterilized, autoclaved brown paper.
Antibiotic Solution Soaking
- Prepare an antibiotic solution using Cefotaxime and add 1 ml to 1000 ml of double-distilled water.[for 1jar 200ul of antibiotic with 200 ml of double distilled water.
- Soak the material in this solution for 20 minutes.
- Used only freshly prepared antibiotic solution.
Final Steps
- Directly add the material to the basal medium .Inoculate the media after 10 days.
- Place the glass bottle at 24°C with a daily photoperiod of 16 hours of light and 8 hours of darkness for tissue culture.

January 2025
After 20 to 25 days, the middle portion of the explant rises upward.

If it rises up to 1 inch, it is ready for passage change.
This is achieved by dividing it into two parts.

Transferring them to another medium or jar.
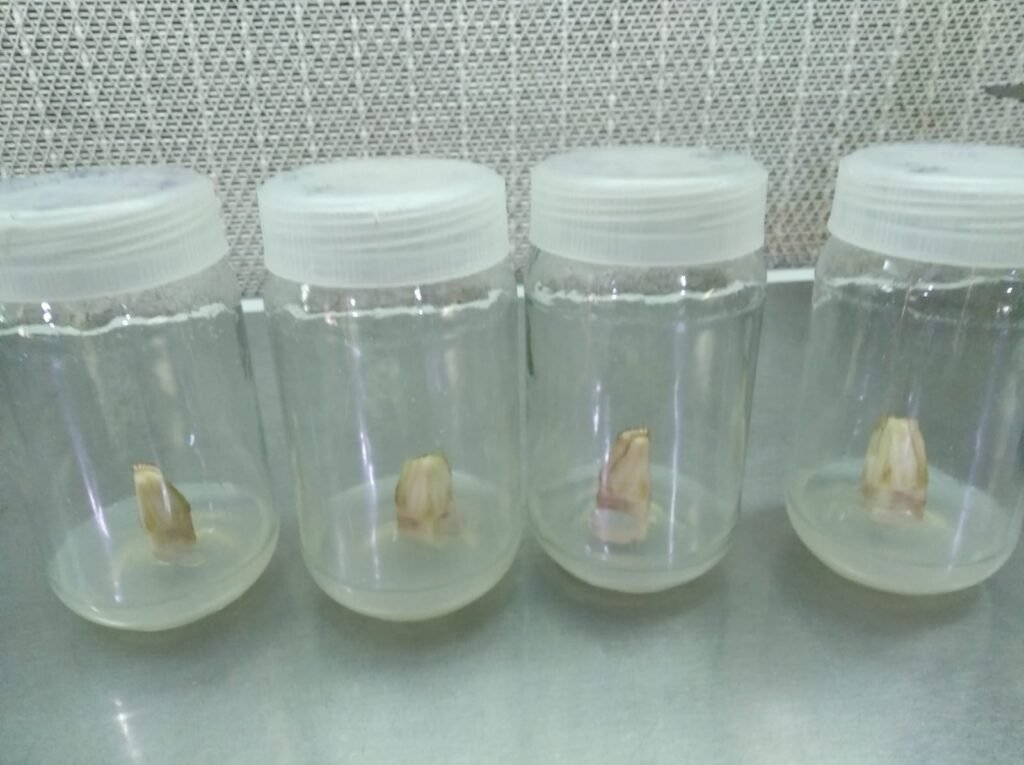
After 15 to 20 days, cut the explant into two equal portions and transfer them to a medium containing MS basal + Harmon (Harmon concentration: [BAP-5mg/L, IAA-0.5 mg/L ]). This step promotes multiplication.
| CHEMICALS / MATERIAL | INITIATION MEDIUM | MULTIPLICATION MEDIUM | ROOTING MEDIUM |
| MS Basal Medium | + | + | + |
| Adenine (200 mg/L) | + | + | + |
| Indole-3-acetic acid | 0.5mg/L | 0.5 mg/L | 0.2mg/L |
| 6-Benzyl amino purine | 10 mg/L | 5mg/L | 2mg/L |
BANANA TISSUE CULTURE DATA (SPREADSHEET) :




