Abstract
In some past years, research in energy storage was developing more efficiently. It is becoming one of the crucial topics for the energy industry. At a time the energy production typically from renewable sources crosses the demand side. This leads to shutting down energy production few examples being hydroelectric and wind turbines. so one of the key issues is the energy storage capacity which is low and costly. This also gets limited by the availability of lithium ore. The other option could be one net through the globe which needs huge investment and political will. Research on this topic is being done to make it more efficient and higher capacity. Electrical energy storage devices like lithium-ion battery, and sodium-ion battery is also one of the leading options, but it is very expensive and its complication makes it inconvenient for users.
Photolytic can use only visible parts of sunlight. leaving 50 % of the energy untapped. On the other side of the story i.e. consumption, there is huge demand of heat and stored heat for room heating and water heating. In our part of the world where room heating requires low or no energy, cooking food, bathing water, and drying farm produced can be shifted to thermal batteries.
We at Vigyan Ashram are focusing on drying of vegetables and fruits as an option for prevacation when excessive production is reported in the harvesting season post-monsoon and early summer as well as the start of the rainy season.
The aim of this project is to design an effective, simple in construction and cheap energy storage device. By constructing a system which heats the sand by excess electric power available in solar power or wind power generation at day time. For that, adding insulation to Thermal Energy Storage (TES) container, directly heating of TES element with heating element without any intermediate medium (air, water, oil, etc.), and using that stored heat for other purpose.
In this project, sand is very cheap, easily available material at any place. Sand is TES material which stores the Heat energy in the form of Sensible heat and it has an average 830 J/Kg °C specific heat capacity which is five time less than that of water which means five times more sand is needed to store same amount of heat as that of water.
Thermal Energy Storage (TES)
Basically, thermal energy is the total of all the kinetic and potential energy of all the particles in
a substance, the average kinetic energy of the particles in a substance is related to temperature linearly, which means as temperature increase, so does the kinetic energy of particles, which lead to an increase in thermal energy. Another Factor of thermal energy is the mass, the more the quantity of a substance (higher mass) the more the number of particles, which means the higher the thermal energy.
Furthermore, the thermal energy flow from one object to another is heat. And heat always flows
from warmer to cooler mediums. Moreover, in nature, some materials heat up or cool down faster than others and this characteristic of materials is referred to as Specific heat, and the physical meaning of specific heat is the amount of heat (Joules) required to raise 1 kilogram of material by 1 degree Celsius or Kelvin. Another characteristic that also affects heat transfer in materials is its thermal conductivity, which is the amount of heat transmitted through a unit thickness of a material, in a direction normal to a surface of unit area
There are many types of TES materials available in market, but sand is a cheap TES material .
Date :28/03/2023
Comparison of specific heat and conductivity of material
| Element | Specific Heat (J/Kg °C) | Conductivity (W/m k) | Density(Kg/m3 ) |
| water | 4184 | 0.59 | 997 |
| Sand | 670 | 0.47-2.5 | 1600 |
| Air | 1005 | 0.026−0.067 | 1.225 |
| Dowtherm | 1558 | 0.1395 | 1063.5 |
Volume/weight required for the sand container:
In discussion we first focused on the amount of sand required for the drying of unit quantity of material
- we took Puran as a sample material for the drying, For the more information of Puran drying , I took the help of Reshama Mam, who is the food lab in-charge.
- In Puran 55 % water is present and the remaining is other ingredients of it.
For calculation we took 1.5 Kg of Puran In 1.5 Kg of Puran 0.825 Kg of water is present, which mean by heating 0.825 Kg of water is to be evaporated.
Amount of heat Required
For ease of calculation we took 1Kg of water instead of 0.825Kg
Mass of water(M)=1Kg
Q evap = m*L
where,
Q evap =Heat required (KJ)
m= Mass of water=1000 gm
L =Latent heat of water=2.260 KJ/gm
Q evap =1000 * 2.260
= 2260 KJ *2 ……………2 (considered 50 % heat loss)
Q evap= 4520 KJ approximately
Q evap= 5000Kj
Amount of Sand required
Q =m*c*(T2-T1)
Where, Q =Heat energy required=5000 KJ
m =Mass of Sand Required(Kg)
c = Specific heat capacity of sand= 0.670KJ/Kg °C.
T2-T1=Change in temperature=150 °C.
5000=m*0.670*150
m=49.75 Kg
For evaporation of 1 kg of water we need around 50 Kg of sand at 200°C. to store heat energy
Date 29/03/2023
Nichrome wire testing at different supply:
| Sr No | Supply | Voltage (Volt) | Current (amp) | Resistance (Ohm) | Temperature ( °C) |
| 1. | AC | 220 | 5.58 | 36 | 190 |
| 2. | AC | 220 | 8.3 | 25 | 248 |
| 3. | AC | 220 | 15.4 | 12.5 | Wire break |
| 4. | AC | 220 | 19.47 | 18 | Fuse break |
| 5. | AC | 220 | 6.8 | Series connection =R1+R2 =13+18 =31 | R1 =400 R2=300 |
| 6. | AC | 220 | 5.1 | Series connection =R1+R2+R3 =10+13+18 =41 | R1=330 R2=305 R3=130 |
| 7. | DC | 12 | 1 | 12 | 40 |
| 8. | DC | 12 | 1 | 36 | No change |
| 9. | DC | 12 | 2 | Parallel Connection =1/R1+1/R2+1/R3 =1/10+1/13+1/18 =4.54 | R1=42 R2=44 R3=50 |
| 10. | DC | 12 | 2.5 | Parallel Connection =1/R1+1/R2+1/R3 =1/10+1/13+1/18 =4.54 | R1=40 R2=43 R3=46 |
| 11. | DC | 20 | 4.43 | Parallel Connection =1/R1+1/R2+1/R3 =1/10+1/13+1/18 =4.54 | R1=53 R2=79 R3=91 |
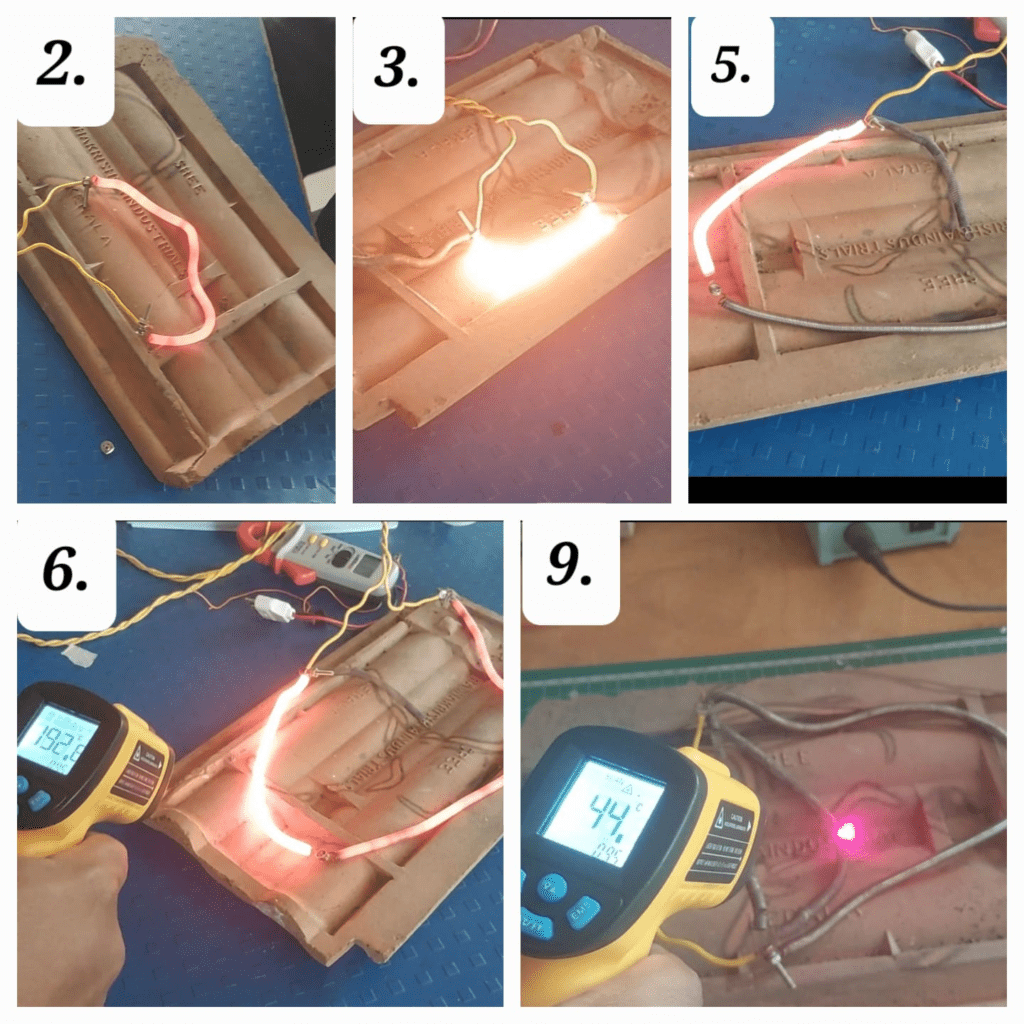
We shown the above data to Dixit sir, sir suggested us to connect ON/OFF switch to heater. The advantage of adding switch is it will not allow the heating element to over heat. At the time of ON condition the heater element get hotter and heats the sand surrounding it, at OFF condition heat absorbed by the sand around the heating element it get transferred to its surround sand by conduction and heating element gets enough time for cooling.
Date:30/03/2023
For more accuracy, and ease of data collection, and ON/OFF of heater circuit we used many electronic devices like k-type Thermocouple for temperature measurement, Arduino for data collection like time and temperature, Relay switch for the ON/OFF of the heater circuit.
Sumedh Bhaiya made one code for the control of conditions in the laptop and then transferred it to the Arduino circuit. As per sir’s suggestion, he controlled the timing of the heater. It will get ON for 3 seconds and OFF for the next 27 seconds with the help of a relay switch. The readings of temperature are noted down after every 5 minutes on the laptop.

Calculating the Specific heat of sand used for readings
For calculating the specific heat of sand I took a measured amount of heated sand and mixes it with a measured amount of cold water. After mixing both together sand releases its heat and its temperature drops, that same amount of heat is absorbed by the cold water and the water’s temperature rises. here we can find the specific heat of sand which is used for testing with the help of Q=m*cp*(T2-T1) formula. Cp of sand is unknown other parameters are known to us.
Q absorb (water)=Q released (sand)
mw *Cpw*(T2-T1)w= ms *Cps*(T2-T1)s
Where, Q =Heat energy is same for both case
mw=Mass of water=88.82 gm
ms=Mass of Sand = 58.45gm
Cpw= Specific heat capacity of water= 4.184J/g °C.
Cps= Specific heat capacity of sand (J/g °C)
(T2-T1)w=Change in temperature of water =(25-22)=3°C.
(T2-T1)s =Change in temperature of sand =(58-25)=33°C
88.82*4.184*(3)=58.45* cps*(33)
Cps=0.577 j/g °C
The calculated specific heat of sand is 0.577 j/g °C.

Date:01/04/2023
Here is the data of temperature readings Vs time,
I have shown the data and calculated the specific heat of sand to Dixit sir. The specific heat is in the specified range as shown in Google. We observed from the data the temperature of sand is constant after 17:17:12.39 PM, it is 161°C. It is not increasing means the heating of sand and heat dissipation of sand to the surrounding is the same, so we stopped the heating of sand and measured the cooling readings of sand. We decided to increase the ON time of heating and decrease the OFF time.
Date:03/04/2023
Density, Bulk density readings, and calculations:
Readings:
- Volume of beaker = 130 ml =1.30*10-4 m3.
- Mass of beaker =17.49 gm.
- Total mass (sand + beaker) =221.21 gm.
- Mass of sand taken = 221.21-17.49 =203.72 gm.
From the above readings, we can find the bulk density of sand, to find its density we need to remove the gaps present in between sand particles so, I added the measured amount of water in the beaker until it will get full. Subtracting the volume of water from the volume of the beaker I got the actual volume of sand.
Density is a concept defined for any substance, while bulk density is only used in cases where the particles or chunks of matter are loosely packed with space for air within.
5. Volume of water occupied in a beaker in the gaps of sand =45 ml =0.45*10-4 m3.
6. Actual volume Occupied by sand =1.30*10-4 – 0.45*10-4 =0.85*10-4m3.
Calculation:
Bulk Density(ρbulk),
ρbulk =M/V,
where, M = Mass of substance (Kg).
V = Volume occupied by substance ( m3).
ρbulk = 0.2037/1.30*10-4
ρbulk = 1566.92 Kg/m3
The Bulk density of Sand is 1567 Kg/m3, which is within the specified range of Google (1520-1680 Kg/m3).

Density(ρ),
ρ =M/V,
=0.2037/0.85*10-⁴
ρ =2396.47 Kg/m³
The density of sand is 2396.47 kg/m³
Date: 04/04/2023
In 2nd trial of the sand battery, we used 3 thermocouples to take temperature readings. We changed the ON OFF timing of heater. It will get ON for 4 seconds and OFF for the next 26 seconds with the help of a relay switch. The readings of temperature are noted down after every 2 minutes on the cloud of wifi. here all readings,

POSITION OF PROBES
Field 1-near Heater temperature
Field 2- Away from the heater
Field 3- At bottom of pot
In the 2nd trial i.e. without insulation the sand is put in a ceramic pot, and heating elements are circulated through the sand. The heating element is a nichrome wire, which gets heat due to its high resistance property of it, when current is passed through it. Here for testing AC power 230-volt power supply is used for the element. There are three temperature sensors are fitted in the setup. Field 1 sensor is fitted near the heater element, field 2 sensor is fitted near to wall side of the pot, and field 3 sensor is fitted at the bottom surface of the sand. these are K types of thermocouple sensors.
The figure shows the graph of temperature versus time heating without insulation. There are different colors are used to indicate the temperature of different sensor positions. At the initial stage, nearly after the one-hour minimum temperature of the sand goes up to 200°C, and at that time the heating element is turned OFF completely. At 181 minute all sand come to an equilibrium temperature, and after that the cooling of sand starts, which we can observe on the graph. The temperature line goes down as time passes. The time required to heat the sand up to 200°C took more time, and at the time of cooling it gets cooled faster.
Date:05/04/2023
In 3rd trial of the sand battery i.e. sand heating with insulation, the sand is put in a ceramic pot, and a 3 Cm thick glass wool insulation layer is provided around it. The thermal conductivity of glass wool insulation is very less, it is around 0.136 W/m k, we used the same 3 thermocouples to take temperature readings. The ON-OFF timing of the heater is the same as the previous one. It will get ON for 4 seconds and OFF for the next 26 seconds with the help of a relay switch. The readings of temperature are noted down after every 2 minutes on the cloud of wifi. here are all readings,
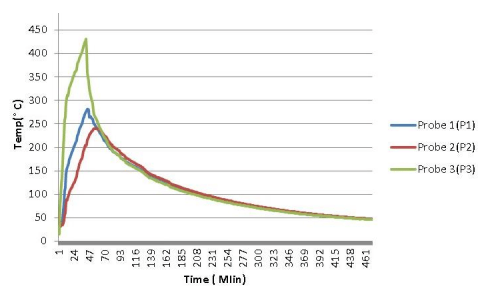
POSITION OF PROBES
Probe 1- Away from the heater element.
Probe 2- At the bottom of the pot.
Probe 3- Near the heating element.
heating elements are circulated through the sand same as in the previous test. The heating. Probe 1 sensor is fitted away from the heater element, probe 2 sensor is fitted at the bottom of the pot, and probe 3 sensor is fitted near the heating element.
The figure shows the graph of temperature versus time heating with insulation. There are different colors are used to indicate the temperature of different sensor positions. At the initial stage, nearly after 47 minutes the minimum temperature of the sand goes up to 250 °C, and at that time the heating element is turned OFF completely. At 70 minute all sand come to an equilibrium temperature, and after that the cooling of the sand starts, which we can observe on the graph. On the graph temperature line goes down as time passes. The time required to heat the sand up to 250°C it took less time, and at the time of cooling it gets cooled slower.
Heating of sand with insulation gave much better results than required. The advantage of adding insulation is that it minimized the heat losses to the surrounding. In the case of heating without insulation, the temperature of sand after equilibrium drops to 50°Cin 2 hours, and in the case of heating with glass wool insulation the temperature of sand after equilibrium i.e. 250°C took 5 and half hours to come at 50°C.
Date: 07/04/2023
Sumedh Bhaiya calculated the best volume-to-surface area ratio on Exel. in which he found a cylinder with less surface area and maximum volume. if the surface area is less then the heat losses to the atmosphere are also less and the volume is maximum to store more sand in it. For these calculations, we used the bulk density of sand. here excel sheet
For 1566.92 Kg/m3 bulk density of sand container with 63816.21 Cm3 of volume is required to store 100 Kgs of sand, and 127632.4 Cm3 of volume to 200 Kgs of sand. The sand which we are using is actually stone crusher sand in which a lot of dust particles are present, these dust particles get melted at the time of heating and stick to the heating element, which leads to damage to the heating element. at the time of testing, we washed that sand with water 3 to 4 times to remove the dust particles from it. We sieved that to separate small particles of sand from big ones.
Doing the same procedure with 100 Kg of sand is very difficult. so, Dixit sir suggested us to use Rangoli as sand for Thermal Energy Storage Material. We brought 1 Kg of sand from the market and tested it for heating. We thought that small particles of rangoli get melted and the same problem will occur with rangoli also, we checked the melting point of rangoli which is 825 °C, and we are going to heat the sand only up to 250 °C so, it will not melt. testing of rangoli is done by heating it.
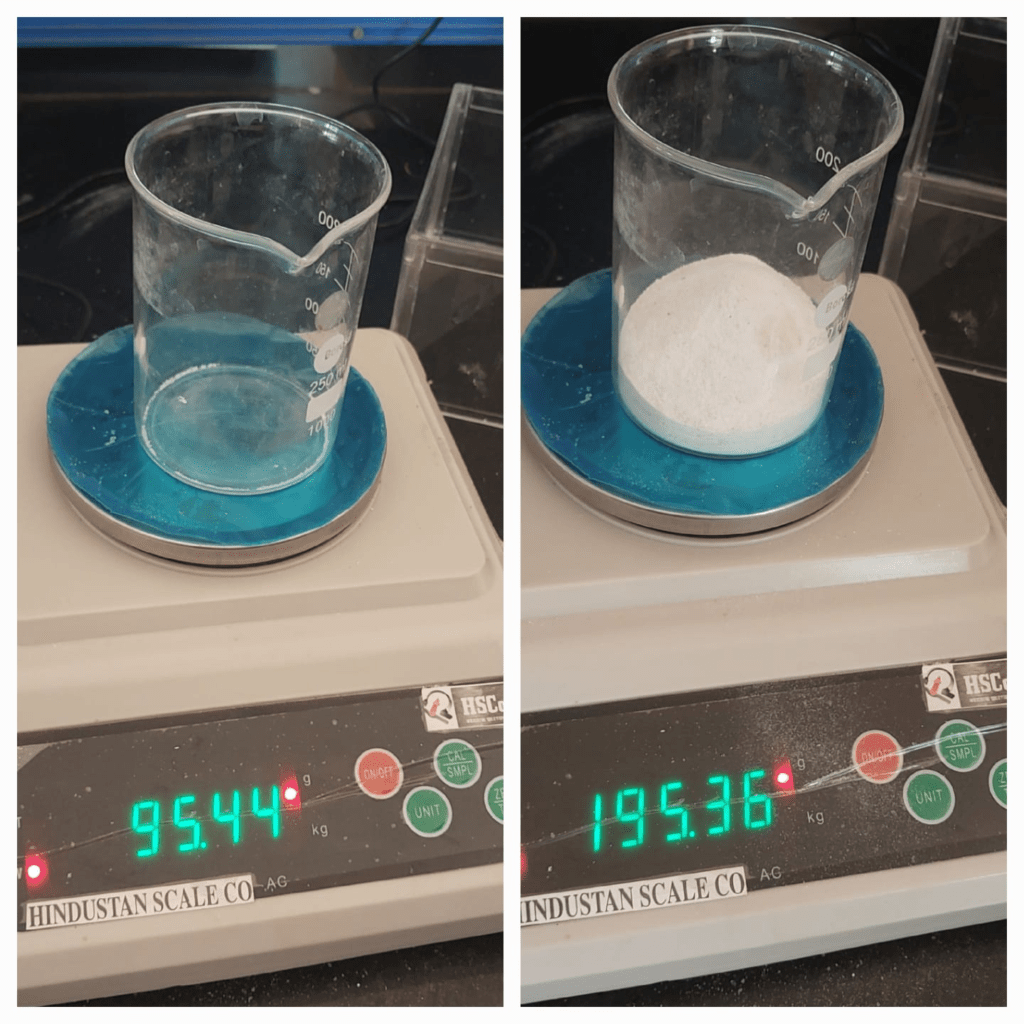
After that, I calculated the density, Bulk density, and Specific heat capacity of Rangoli. The calculations are as follows.
Calculation:
Bulk Density(ρbulk),
ρbulk =M/V,
where, M = Mass of substance (Kg).
V = Volume occupied by substance ( m3).
ρbulk = 0.201058/1.34*10-4
ρbulk = 1571.49Kg/m3
The Bulk density of rangoli is 1567 Kg/m3
Density(ρ),
ρ =M/V,
=0.2037/0.98*10-⁴
ρ =2148.77 Kg/m³
The density of rangoli is 2396.47 kg/m³
Specific heat of Rangoli:
Here, I used the same procedure as that of sand to calculate the specific heat of Rangoli.
Q absorb (water)=Q released (sand)
mw *Cpw*(T2-T1)w= ms *Cps*(T2-T1)s
Where Q =Heat energy is the same for both cases
mw=Mass of water=100 gm
ms=Mass of Sand = 100gm
Cpw= Specific heat capacity of water= 4.184J/g °C.
Cps= Specific heat capacity of sand (J/g °C)
(T2-T1)w=Change in temperature of water =(42-28)=14°C.
(T2-T1)s =Change in temperature of sand =(115-42)=73°C
100*4.184*(14)=100* cps*(73)
Cps=0.802 j/g °C
The calculated specific heat of Rangoli is 0.802 j/g °C.
ρbulk =M/V,
Where, M = Mass of substance (Kg).
V = Volume occupied by substance (m3).
Bulk density of rangoli =1571.49Kg/m3
Mass of sand =200 Kg
Volume of sand = 0.12726775 m3 = 127267.75 Cm3
Table 3.1 Volume to surface area ratio (1.1 to 14.33)
| Radius (Cm) | Height (Cm) | Volume (Cm3) | Vol: SA |
| 2 | 10123.5712 | 127267.752 | 1.99921008 |
| 5 | 1619.77139 | 127267.752 | 4.96932085 |
| 10 | 404.942848 | 127267.752 | 9.52934847 |
| 20 | 101.235712 | 127267.752 | 14.3357102 |
| 30 | 44.9936498 | 127267.752 | 12.856106 |
| 40 | 25.308928 | 127267.752 | 9.61321266 |
| 50 | 16.1977139 | 127267.752 | 6.96989354 |
| 60 | 11.2484124 | 127267.752 | 5.1421936 |
| 70 | 8.26413976 | 127267.752 | 3.90175119 |
| 80 | 6.327232 | 127267.752 | 3.04326931 |
| 90 | 4.99929442 | 127267.752 | 2.43209845 |
| 100 | 4.04942848 | 127267.752 | 1.98453312 |
| 110 | 3.34663511 | 127267.752 | 1.6482445 |
| 120 | 2.81210311 | 127267.752 | 1.38976752 |
| 130 | 2.39611153 | 127267.752 | 1.18711553 |
The table shows the different combinations of density 63633.88 Cm3 with radius 2 Cm to 130 Cm by and height 2.396 Cm to 10123.57 Cm. Here the maximum Volume to the surface area ratio is between 4.9 and 5.1.
Table Volume to surface area ratio (10.32 to 14.11)
| Radius (Cm) | Height (Cm) | Volume (Cm3) | Vol: SA |
| 11 | 334.663511 | 127267.752 | 10.32148932 |
| 12 | 281.210311 | 127267.752 | 11.05638837 |
| 13 | 239.611153 | 127267.752 | 11.72746307 |
| 14 | 206.603494 | 127267.752 | 12.32909564 |
| 15 | 179.974599 | 127267.752 | 12.85688363 |
| 16 | 158.1808 | 127267.752 | 13.30782498 |
| 17 | 140.118633 | 127267.752 | 13.68042419 |
| 18 | 124.982361 | 127267.752 | 13.97471426 |
| 19 | 112.172534 | 127267.752 | 14.19219673 |
| 20 | 101.235712 | 127267.752 | 14.33571022 |
| 21 | 91.8237751 | 127267.752 | 14.40924287 |
| 22 | 83.6658777 | 127267.752 | 14.41770771 |
| 23 | 76.5487426 | 127267.752 | 14.36670048 |
| 24 | 70.3025778 | 127267.752 | 14.26225784 |
| 25 | 64.7908557 | 127267.752 | 14.11063087 |
The table shows the combinations of density 63633.88 Cm3 within the range of 10.3 and 14.41 Volume to Surface area ratio with a radius of 11 Cm to 25 Cm and height 64.79 Cm to 334.66 Cm.
Table 3.3 Volume to surface area ratio (14.39 to 14.42)
| Radius (Cm) | Height (Cm) | Volume (Cm3) | Vol: SA |
| 20.7 | 94.5046204 | 127267.752 | 14.39425412 |
| 20.8 | 93.5981065 | 127267.752 | 14.39991037 |
| 20.9 | 92.7045736 | 127267.752 | 14.40490488 |
| 21 | 91.8237751 | 127267.752 | 14.40924287 |
| 21.1 | 90.95547 | 127267.752 | 14.41292962 |
| 21.2 | 90.0994233 | 127267.752 | 14.41597047 |
| 21.3 | 89.2554053 | 127267.752 | 14.41837085 |
| 21.4 | 88.4231916 | 127267.752 | 14.42013623 |
| 21.5 | 87.6025631 | 127267.752 | 14.42127216 |
| 21.6 | 86.7933059 | 127267.752 | 14.42178422 |
| 21.7 | 85.9952108 | 127267.752 | 14.42167807 |
| 21.8 | 85.2080734 | 127267.752 | 14.42095943 |
| 21.9 | 84.4316941 | 127267.752 | 14.41963404 |
| 22 | 83.6658777 | 127267.752 | 14.41770771 |
| 22.1 | 82.9104335 | 127267.752 | 14.4151863 |
The table shows the combinations of density 63633.88 Cm3 within the range of 14.39 and 14.4217 Volume to Surface area ratio with a radius of 20.7 Cm to 22.1 Cm and height of 82.91 Cm to 94.50 Cm.
From all these calculations, a cylinder with a radius of 22 Cm and height of 83 Cm is best for volume to surface area ratio.
Note File:





