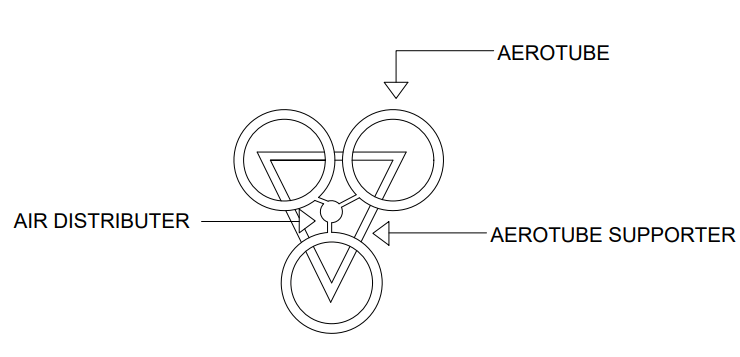
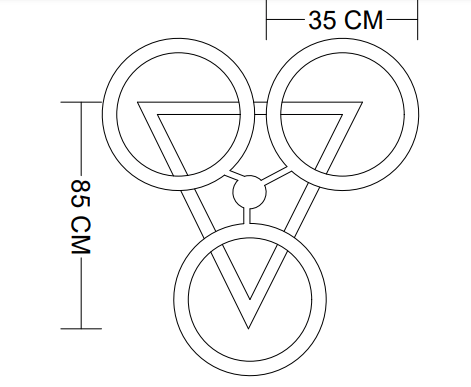
Introduction
Aeration is the process by which air is circulated through, mixed with or dissolved in a liquid or substance. Aeration is used in liquids, soils and foods to improve quality and reduce contamination. In industrial water conditioning, one of the major objectives of aeration is to remove carbon dioxide. Aeration is also used to oxidize soluble iron and manganese to insoluble precipitates. It can also reduce ammonia and hydrogen sulfide (stripping), and is an effective method of bacteria control. Selecting appropriate manifold for aeration to do different experiments then get results from them then decide which one is better for Aeration grey water.
Objectives
- To conduct different experiments on grey water at Vigyan Ashram
- To collect data and analyze it on that basis suggest proper Aeration Manifold.
Process flow diagram
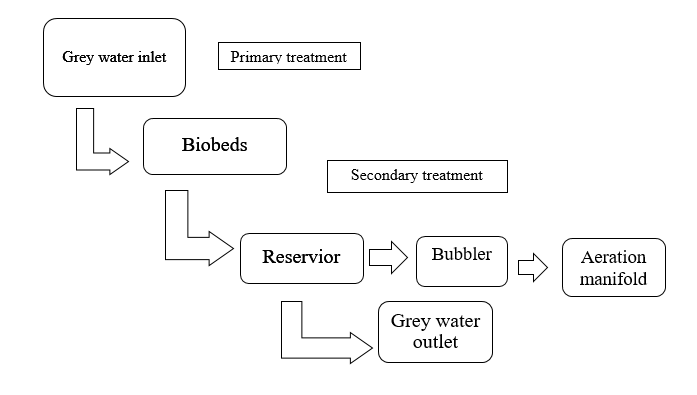
In this process I only work for secondary treatment like aeration Manifold and data collection. As below different experiments and data
Experiments
1. MANIFOLD MAKING EXPERIMENTS
- EXPERIMENT NO 1
Date 24/02/2022
Aim
we have to blow the same air from every hole of manifold.
Procedure
We did an experiment on dated 24/02/22. in that experiment we takes 1.5 In PVC pipe and one ventilation fan. we close the one end of that PVC pipe with the help of polythene. we did 3 holes on that pipe with the distance of 1 ft. that all holes are different diameters 4mm, 6mm and 8 mm. then we blow the air in that pipe and we took reading on anemometer (m3/min)
Observation
Fan blowing air through the pipe 435 (m3/min)
In 4mm hole we got 55.3
In 6mm hole we got 78.6
In 8mm hole we got 106.3
Conclusion
This experiment went completely wrong because what we are expected in results we didn’t get it.
Result
This experiment was proved wrong.


- EXPERIMENT NO 2
DATE 11/03/2022
Aim
we have to blow the same air from every hole of manifold.
Procedure
We re-arrange the setup. then we did small modification like hole diameter and tee joints also we used laser cutter machine to cut the MDF sheet to fill the gap in between tee and pipe and we closed the one end. and put the anemometer on top handle and take the readings on it.
Observation

Conclusion
In this experiment we tried to circulate same air flow each hole
Result
we got different flow rate from every hole its not distributes equally.


Date 25/03/2022
on this day I had small experiment on it as follows
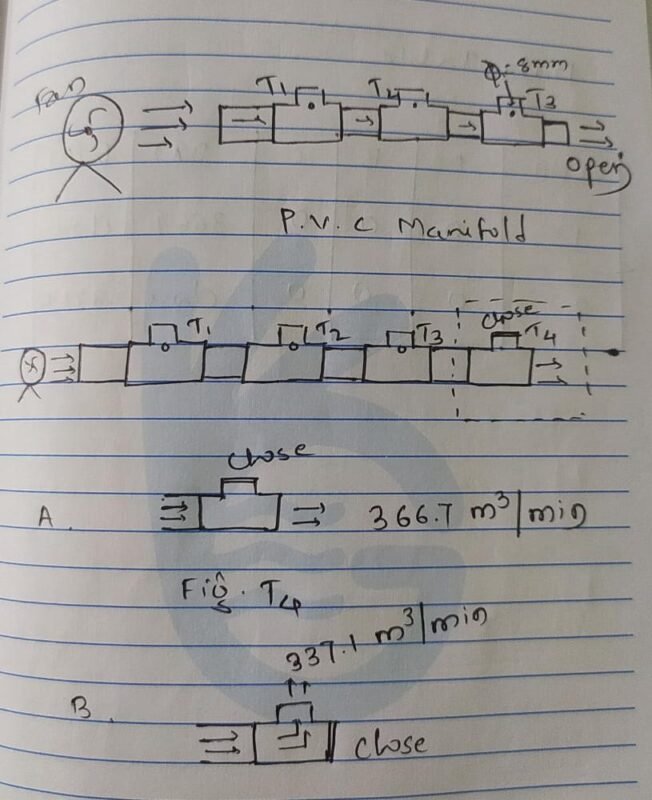
Result
we get some resistance when it flows to vertical direction when it comes to horizontal direction flow rate will increases.
- Till now these experiments are not works to get Idea to design a proper manifold
- We had brain storming session with Praad sir and Dixit sir. In this brain storming session were discussed many designs of manifold and refer the other some designs on that basis I got other experiments like to determine the COD from bubbled water and without bubbled water.
2. Blower bubbling and without bubbling experiments
Date 07/04/2022 to 08/04/2022
I have to learn the how to determine the COD by titration method.
Materials for COD test
- Potassium Dichromate (K2Cr2O7).
- Mercuric sulphate (HgSO4)
- Sulfuric acid (H2SO4)
- Ferrous ammonium sulphate (FAS)
- Ferron indicator
- Potassium hydrogen phosphate (KHP)
- 250 ml Erlenmeyer flask
- Reflux condenser
- COD tubes
- Measuring cylinder
- Analytical balance
- COD tube stands
- Hand gloves
Preparation of chemicals
- Preparation of 0.25 N potassium dichromate solution: –
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask. - Preparation of Ammonium Ferrous Sulphate solution: –
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid. - Preparation of Potassium Hydrogen Phosphate solution: –
0.425 gm. of dried KHP powder dissolved in 1000 ml distilled water in 1000 ml
volumetric flask. (Dried this powder for 2 hours in oven)
Procedure
- Take 0.4 gm. AgSo4 ( Silver Sulphate ) in COD tubes.
- Added 20 ml sample in each COD tube.
- Added 20 ml distilled water in each COD tube.
- Added stones (crush on beads) 1 or 2 pieces.
- Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
- Added 30 ml concentrated sulfuric acid slowly along with swirling.
- If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
- Kept COD tubes in COD apparatus to digest the sample..
- Attached the condenser & set 150 Degree Celsius temperature.
- Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
- After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
- Took the above sample in a conical flask. Added 150 ml distilled water.
- Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
- A-Reading blank solution.
- B-Reading with sample solutions
- N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
Calculation of COD
| Sample | Readings | COD (mg/lit) |
| Blank | 10.4 | – |
| KPH | 4.6 | 529 |
| Black water | 8.1 | 2340 |
| Grey water | 8.9 | 612 |
| Sample | Readings | COD (mg/lit) |
| Blank | 10.1 | – |
| Tap water | 9.5 | 61.2 |
| Black water | 8.5 | 1632 |
| Grey water | 9.4 | 714 |
The sample of grey water and black water is diluted with distilled water and dilution factor is considered in calculation. On first day, sample of grey water was collected from grey water system near Dixit Sir’s house and on second day, it was collected from grey water system near the food lab. The COD of black water is 2340 mg/lit and we have reduce it.
Conclusion
we got many results on black water, grey water and tap water. and I learnt how to check the COD by using Titration method.
DATE 13/04/2022
- We had brainstorming session with Dr Dixit and Prasad Patil sir about air distribution designs on design material cost etc.
- in this discussion we decided to take 3 reservoir tanks put into the each tank in grey water and starts to bubbling the water like one is aerotubes second one is only pipe and third one is without bubbled water.
Date 16/04/2022
on this dated we got bubbler we started working on our experiment.
DATE 19/04/2022 TO 23/04/2022
we started work on required material and prepared BOM of its.
- EXPERIMENT NO 1
Aim
we have to check the COD from aerotube bubbled grey water, without bubbled grey water and only pipe through bubbled water.
Apparatus
1.Potassium Dichromate (K2Cr2O7).
2.Mercuric sulphate (HgSO4)
3.Sulfuric acid (H2SO4)
4.Ferrous ammonium sulphate (FAS)
5.Ferron indicator
6.Grey water Sample
7.250 ml Erlenmeyer flask
8.Reflux condenser
9.COD tubes
10.Measuring cylinder
11.Analytical balance
12.COD tube stands
13.Hand gloves
Preparation of chemicals
- Preparation of 0.25 N potassium dichromate solution
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask. - Preparation of Ammonium Ferrous Sulphate solution
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid.
Procedure
- Take 0.4 gm. AgSo4 ( Silver Sulphate ) in COD tubes.
- Added 20 ml sample in each COD tube.
- Added 20 ml distilled water in each COD tube.
- Added stones (crush on beads) 1 or 2 pieces.
- Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
- Added 30 ml concentrated sulfuric acid slowly along with swirling.
- If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
- Kept COD tubes in COD apparatus to digest the sample..
- Attached the condenser & set 150 Degree Celsius temperature.
- Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
- After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
- Took the above sample in a conical flask. Added 150 ml distilled water.
- Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
- A-Reading blank solution.
- B-Reading with sample solutions
- N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
Calculation
| Sample | Readings | COD (mg/lit) |
| Blank | 9.6 | – |
| Grey water | 8.4 | 1224 |
| Aerotube bubbled water | 8.6 | 1020 |
| Pipe through bubbled water | 8.8 | 816 |
Conclusion
Aerotube bubbled water will less drops the COD as compare to only Pipe through bubbled water.



Date 27/04/2022 to 03/05/2022
we arrange another setup for to determine the COD ,we take 1001lit IBC tanks and to fit blower on it one is only pipe another one is aerotube then started to blowing to continuously 8 hours and take reading on it
- EXPERIMENT NO 2
Aim
we have to check the COD from 1000 lit IBC tank it will drops or not
Apparatus
1.Potassium Dichromate (K2Cr2O7).
2.Mercuric sulphate (HgSO4)
3.Sulfuric acid (H2SO4)
4.Ferrous ammonium sulphate (FAS)
5.Ferron indicator
6.Grey water Sample
7.250 ml Erlenmeyer flask
8.Reflux condenser
9.COD tubes
10.Measuring cylinder
11.Analytical balance
12.COD tube stands
13.Hand gloves
Preparation of chemicals
- Preparation of 0.25 N potassium dichromate solution
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask. - Preparation of Ammonium Ferrous Sulphate solution
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid.
Procedure
- Take 0.4 gm. AgSo4 ( Silver Sulphate ) in COD tubes.
- Added 20 ml sample in each COD tube.
- Added 20 ml distilled water in each COD tube.
- Added stones (crush on beads) 1 or 2 pieces.
- Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
- Added 30 ml concentrated sulfuric acid slowly along with swirling.
- If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
- Kept COD tubes in COD apparatus to digest the sample..
- Attached the condenser & set 150 Degree Celsius temperature.
- Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
- After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
- Took the above sample in a conical flask. Added 150 ml distilled water.
- Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
- A-Reading blank solution.
- B-Reading with sample solutions
- N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
Calculation
| Sample | Readings | COD mg/lit |
| Blank | 10.4 | – |
| Food lab IBC grey water | 9.2 | 1224 |
| Food lab IBC grey water after bubbling through pipe | 9.4 | 1020 |
| Blank | 10 | – |
| Fish tank IBC water | 9.2 | 714 |
| Fish tank IBC water after bubbling through aerotube | 9.4 | 612 |
Conclusion
As compare to pipe bubbling aerotube bubbling less drops the COD.


- On these observation basis we have decided to do little changes in the experiments like take same grey water and do experiments on it .
- This brainstorming session done with Kulkarni sir and Dixit sir on Dated 04/05/2022
DATE 27/05/2022
We arranged another bubbling setup for grey water bubbling like 1000 lit 2 IBC tanks and 58 W bubbler for blowing and aerotube manifold for aeration
- EXPERIMENT NO 3
Aim
We have to determine initial COD of grey water then bubble the grey water 5 hour and determine the COD note down the reading and after 8 hour bubble the grey water determine the COD.
Bubbling Setup



Apparatus
1.Potassium Dichromate (K2Cr2O7).
2.Mercuric sulphate (HgSO4)
3.Sulfuric acid (H2SO4)
4.Ferrous ammonium sulphate (FAS)
5.Ferron indicator
6.Grey water Sample
7.250 ml Erlenmeyer flask
8.Reflux condenser
9.COD tubes
10.Measuring cylinder
11.Analytical balance
12.COD tube stands
13.Hand gloves
Preparation of chemicals
- Preparation of 0.25 N potassium dichromate solution
12.25 gm. potassium dichromate powder dissolved in 1000 ml distilled water in 1000
ml volumetric flask. - Preparation of Ammonium Ferrous Sulphate solution
24.5 gm. Ammonium Ferrous sulphate powder dissolved in 250 ml distilled water in
250 ml distilled water in 250 ml volumetric flask. Add 5ml Sulphuric acid.
Procedure
- Take 0.4 gm. AgSo4 ( Silver Sulphate ) in COD tubes.
- Added 20 ml sample in each COD tube.
- Added 20 ml distilled water in each COD tube.
- Added stones (crush on beads) 1 or 2 pieces.
- Added 10 ml (0.25 N) K2Cr2O7 Potassium dichromate solution.
- Added 30 ml concentrated sulfuric acid slowly along with swirling.
- If the solution turns green more known quantities of K2Cr2O7 solution is needed to add.
- Kept COD tubes in COD apparatus to digest the sample..
- Attached the condenser & set 150 Degree Celsius temperature.
- Kept for 2 hours filled COD tubes in COD apparatus for 150 degree Celsius.
- After 2 hours switched it off & removed COD tubes & cooled sample for room temperature.
- Took the above sample in a conical flask. Added 150 ml distilled water.
- Titration: – Titrated above solution with 0.1 N FAS (ferrous ammonium sulphate) solution by using Ferron indicator (add 3-4 drops). The end point of this titration is blue green to reddish brown.
Formula of COD
COD = {(a-b) X N X 8000}/ml of sample solution
Where,
- A-Reading blank solution.
- B-Reading with sample solutions
- N- Normality of FAS solution
Dilution factor = ( ml of sample + distilled water )/ ml of sample
Calculations
Initial COD of grey water

After 5 hour bubbling the grey water

After 8 hour bubbling the grey water

Observations table
| Time ( hours ) | Aerotube ( mg/lit ) | Without Aerotube ( mg/lit ) |
| 0 | 1222 | 1222 |
| 5 | 204 | 612 |
| 8 | 102 | 306 |
Conclusion
- In this experiment we get results what were expected COD dropped in less time like 5 hour bubbling time period.
- Grey water must have 250 mg/lit COD as per government norms if used this manifold it will comes 250 to 150 mg/lit COD as per our experiment.
3. DESIGN OF MANIFOLD