Need of project :- – ETP plant of SI group is working properly but sometimes it has disturbance in microbial flora which affects the COD and BOD of outlet. To avoid this problem we have to develop culture such that whenever microbial flora of ETP plant gets disturbed we can add preserved culture which can control COD and BOD of outlet.
Objectives :-
- To isolate microorganisms from given culture sample.
- To set SOP for mass multiplication of culture isolated from sample bought from ETP plant of SI group.
Methodology :- We collect working culture from outlet of ETP plant then we will isolate good consortia from sample. Isolated consortia we will preserve and maintain quality of culture and develop SOP for multiplication of consortia. Whenever we need that culture, we will multiply it in lab or field itself.
Duration of Project :- 3 months
Date- 01-08 -2021
Procedure :-
Part A :- Aeration of Sample :-
The ETP plant sample was given to Vigyan Ashram , to keep the culture aerated we used rotary shaker . The culture was kept on rotary shaker for 5 days to maintain CFU ( Colony Forming Units ) of culture .

Date:- 07 -08-2021
PART B :- Preparing plates for isolation of existing culture .
Preparation of NA ( Nutrient Agar ) :- Nutrient Agar is used for the cultivation of bacteria and for the enumeration of organisms in water, sewage, feces and other materials.
Composition of nutrient agar :-
| Ingredient | 1 L | 500 mL |
|---|---|---|
| Yeast extract | 2 g | 1 g |
| Peptone | 5 g | 2.5 g |
| Sodium chloride (NaCl) | 5 g | 2.5 g |
| agar | 15 g | 7.5 g |
Preparation of PDA ( Potato Dextrose Agar ) :-
Potato Dextrose Agar, often notated as PDA, is a common microbial growth media made from an infusion of potato and dextrose. . The nutritionally rich base from the potato infusion encourages mold sporulation and pigment production in certain dermatophytes while dextrose supports a general growth of microorganism.
Composition of PDA :-
| Dextrose | 20 g |
| Potato extract | 4 g |
| Agar | 15 g |

Date :- 20-08-2021
PART C :- Isolation of microorganisms from sample by using spread plate technique .
- The plates of NA and PDA were poured .
- 10 ml sample was taken in sterile test-tube and dilutions were made using serial dilution technique .
- These dilutions were spread on NA and PDA plates by using spread plate technique .
- These plates were incubated for 5 days to observe growth .


CONTAMINATION WAS SEEN DUE TO IMPROPER STERILIZATION AND IMPROPER HANDLING OF MEDIA
NOTE :- This contamination was seen due to over incubation of plates or the culture given from SI group itself contains eggs .
Date :- 26-08-2021
This procedure was repeated again and incubation period was reduced to 3 days . After incubation of 3 days normal growth was seen which was expected .
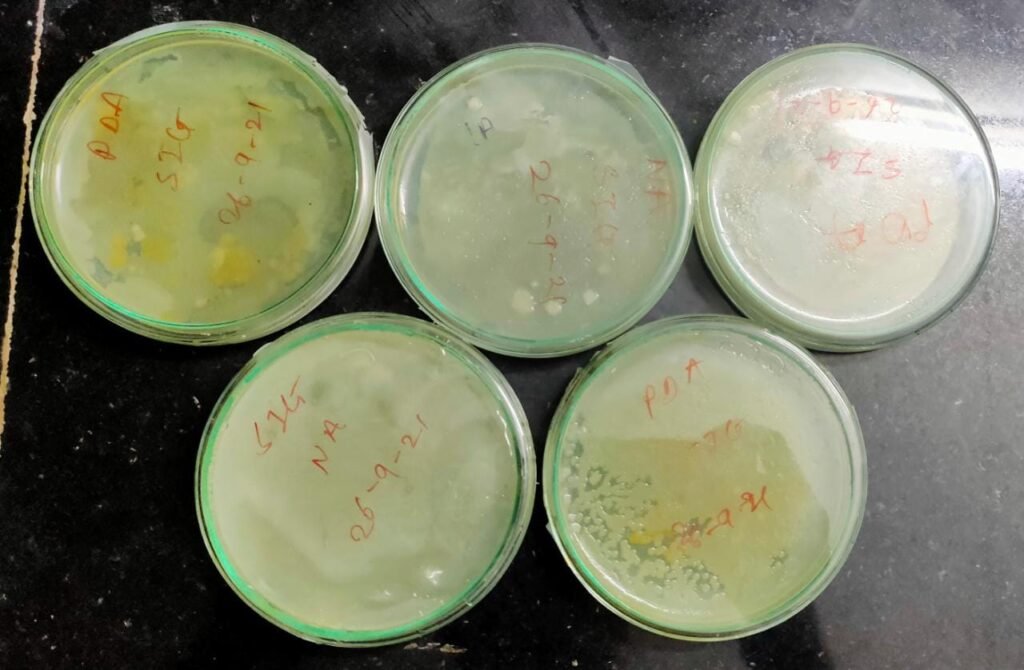
Date:- 5- 09 – 2021
PART D :- SCRAPPING THE GROWTH AND TRANSFERRING IT TO NUTRIENT BROTH MEDIUM FOR MASS MULTIPLICATION
composition nutrient broth medium for mass multiplication
| Ingredient | 1 L | 500 mL |
|---|---|---|
| beef extract | 1 g | 0.5 g |
| Yeast extract | 2 g | 1 g |
| Peptone | 5 g | 2.5 g |
| Sodium chloride (NaCl) | 5 g | 2.5 g |
- The growth on petri plates was transferred to sterile nutrient broth for mass multiplication.
- This broth was kept on rotary shaker for 5 days.
- After 2 days sugar source (sucrose) was added for optimum growth

Now ,3 nutrient broth medium flasks were used for mass multiplication of culture , reason was to isolate bacteria and fungus from the culture .
| Sr no. | Culture added to nutrient broth medium |
| 1 | Flask 1 ( 10 ml of sample from ETP plant ) |
| 2 | Flask 2 (Isolated bacterial growth from NA plates ) |
| 3 | Flask 3 (Isolated fungal growth from PDA plates ) |
Now we have 3 cultures , for optimum growth of these cultures we provided sugar source for best growth .
Sucrose was added to these flasks for optimum growth of culture on 2nd day of incubation .

Date :- 15-09-2021
PART E :- SUBCULTURING OF CULTURES
After 5 days of incubation , the nutrients in the nutrient broth will be consumed by respective microorganisms so there is a need to do sub culturing of these cultures , so that they can grow optimally .
Now we have used , 200 ml NB broth in which composition of nutrients is doubled .
| Ingredient | 200 ml |
| Beef extract | 0.50 g |
| Yeast extract | 2.50 g |
| Peptone | 4 g |
| Sodium chloride (NaCl ) | 4 g |

PREPARATION PDA BROTH FOR FUNGUS CULTURE :-
Procedure :-
- Take approximately 4 potatoes and weigh it .
- Wash it and boil and remove peels and oven dry it at 60 degree for 4-5 hours .

- After drying , grind it using grinder and make fine powder .
- Add 50 g of potato infusion powder to 200 ml D/W.
- Add 10 g of dextrose to it . And your broth is ready .
Date:- 28-09-2021
PART F :- MASS MULTIPLICATTION OF CULTURE
WHAT IS MASS MULTIPLICATION ?
Mass production is the growing cultures in large volume , to get your desired products in large quantity .
Now we have 3 cultures , now its time to mass multiply this cultures .
so for mass multiplication , we used jaggery as sugar source and urea as nitrogen source.
PROCEDURE FOR MASS MULTIPLICATION :-
- First take 2 kg of jaggery in bucket and dissolve it in water.


- Then take 80 litre drum and add 60 liters of water in it.
- Now add 10 liters of jaggery dissolved water in it and bring volume up to 70 liters.

- Then add 10 grams of urea to it and add 10 grams of chill mix as micronutrient source.

- Keep this culture for 5 days.
- Then our 80 liters of culture is ready for trials .


Now , this culture will be tested to reduce COD of given sample .
COD TRIALS
DATE :- 05-10-2021
WHAT IS COD ?
In Environmental chemistry, the chemical oxygen demand (COD) is an indicative measure of the amount of oxygen that can be consumed by reactions in a measured solution.
It is commonly expressed in mass of oxygen consumed over volume of solution which in SI units is milligrams per litre (mg/L).
A COD test can be used to easily quantify the amount of organics in water.
The most common application of COD is in quantifying the amount of oxidable pollutants found in surface water (e.g. lakes and rivers or wastewater.
MECHANISM OF COD :-
Most of the organic matters are-destroyed when boiled with a mixture of potassium dichromate and sulphuric acid producing carbon dioxide and water. A sample is refluxed with a known amount of potassium dichromate in sulphuric acid medium and the excess of dichromate is titrated against ferrous ammonium sulfate. The amount of dichromate consumed is proportional to the oxygen required to oxidize the oxidable organic matter.
Reagents :-
- 0.25 M Ferrous Ammonium Sulfate (FAS) :- Dissolve 98 gm Ferrous Ammonium Sulfate + 800 ml distilled water +20 ml Sulfuric acid and make it up to 1000 ml with distilled water.
- 0.25 N K2Cr2O7 :- Dissolve 12.25 gm K2Cr2O7 in 1000 ml volumetric flask and fill Distilled water up to mark.
- Potassium hydrogen phthalate (KHP) :- 0.425 gm Dried powder KHP in 1000ml D/W.
- Mercuric Sulfate.
- Ferroin Indicator.
- Samples to checked ( SI group , Phenol )
Procedure :-
1. Take 0.4 HgSO4 ( Mercuric Sulfate) in COD test tube.
2. Add 20 ml KHP sample and add 20 ml D/W in it, Mix well. Run Blank with D/W simultaneously.
3. Add 2-3 cumic stones or glass beads in it.
4.Add 10 ml 0.25 N K2Cr2O7 solution.

5.Add 30 ml of H2SO4.After addition of H2SO4, If color changes to green then it goes up to limit of our COD apparatus so we add know quantity of K2Cr2O7 or we make dilution of test sample. And keep tubes for reflux at 150 degree for 2 hours .


6.Take out COD tubes for cooling.
7.After cooling ,Take all solution in conical flask and add 150 ml D/W and add 5 drops of ferroin indicator. and titer against FAS.
8. Add drop by drop FAS up to color changes from yellowish orange to brick red color. Note down reading. and calculate.

Observation table :-
| solution | Burette reading | COD ( mg /lit ) |
| Blank | 9.8 ml | – |
| Phenol ( 1:50 ) | 7 ml | 1000 mg /lit |
| SI group inlet sample | 6.4 ml | 94000 mg /lit |
| Phenol ( 1:20) | 9.6 ml | 5600 mg /lit |
Hence , initial COD of SI inlet sample was 94000 mg /lit .
This was the initial COD of sample given , now we will inoculate our culture developed in it and will incubate it for 5 days , after 5 days we will again estimate COD .
Now , we got initial COD of sample , it was 94000 which is too high for microbial culture to multiply in it .
So , we diluted the sample 6 times and 15 times as per requirement .
Now , we had 2 samples which were diluted and we inoculated 100 ml of culture to it and initial readings were taken for COD to check reduction in COD in 5 days .
To check COD reduction we should know initial COD of diluted sample .

hence , initial sample of was
| solution | burette reading | COD reading (mg/lit ) |
| Blank | 10 ml | 00 |
| Flask 1 ( 1:15 ) | 7 ml | 6000 mg /lit |
| Flask 2 (1:6) | 4.9 ml | 10200 mg / lit |
Initial COD was calculated and culture was inoculated in it , and flasks were incubated and COD readings were taken per day to check COD reduction .
DATE :- 10-10-2021
| Solution | Burette reading | COD reading ( mg/lit ) |
| Blank | 10.8 ml | 00 |
| Flask 1 ( 1:15 ) | 9.4 ml | 2800 mg /lit |
| Flask 2 (1:6) | 8.5 ml | 4600 mg /lit |
DATE – 12-10-2021
The initial COD of sample after dilution is 10200 in 1:6 10200mg/lit which was dropped to 4600 mg /lit in 24 hours .
and COD of 1:15 diluted sample was 6000 mg /lit and was dropped to 2800 mg /lit after 24 hours .
| Solution | Burette reading | COD reading ( mg/lit ) |
| Blank | 10 ml | 000 |
| Flask 1 ( 1:15 ) | 9.2 ml | 1600 mg /lit |
| Flask 2 (1:6) | 8.4 ml | 3200 mg /lit |
As per observation table , we can see the COD is consistently decreasing with increase in incubation period .
COD was decreased from 10200 mg /lit to 3200 mg /lit in 3 days .
PRESERVATION OF CULTURE :-
DATE :- 20-10-2021
We got to know , our culture was contributing to decrease rapidly , hence we decided to preserve this culture by using suitable preservation techniques .
The method of preservation used was applying glycerin to plates and slants .
DATE :- 21-10–21
Why to use glycerin ?
The addition of glycerol stabilizes the frozen bacteria, preventing damage to the cell membranes and keeping the cells alive. A glycerol stock of bacteria can be stored stably at -80°C for many years.
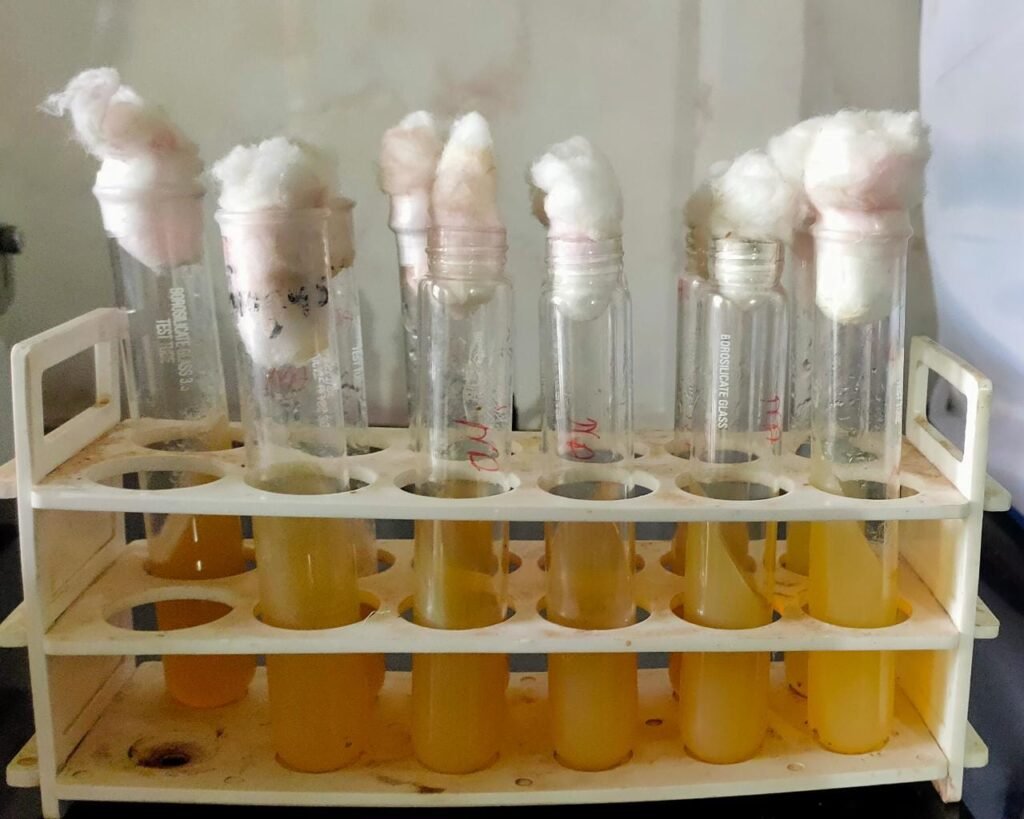
We prepared 5 slants , 5 plates of above culture and preserved it using glycerin for further use .

CONCLUSION OF PROJECT
All the objectives of above project are completed and COD reduction was seen as expected .
| solution | Initial COD | COD after 5 days |
| flask 1 (1:6 ) | 10200 mg/lit | 3200 mg/lit |
| flask 2 (1:15) | 6000 mg/lit | 1600 mg/lit |





