Need of project :- In Vigyan Ashram , we were using standard kits like Nagarjuna , Prerana soil testing kits which were used for quantitative as well as qualitative determination. Now there’s a need to set up our laboratory with these Standard Operating Procedures .And to make it available open source through Soil Manual.
Estimation of Carbon from soil sample by Walkey -Black method
Introduction:- Estimates of organic carbon are used to assess the amount of organic matter in soil . The method measures the amount of organic carbon in plant and animal remains including soil humus .
We perform this experiment 6 times in our lab. while performing the experiment some problems were observed in preparation of chemicals , handling laboratory instruments , lack of knowledge in standardization of chemicals were observed . Then we tested 3 different soil samples in our lab with constant repeatability then final SOP for Organic Carbon was obtained which is mentioned below .
Date – 27-06-2021
Aim :- To estimate organic carbon from soil sample by walkey black method
Equipment:-
1. 500-mL Erlenmeyer flasks.
2. 10-mL pipette.
3. 10-and 20-mL dispensers.
4. 50-mL burette.
5. Analytical balance.
6. Magnetic stirrer.
7. Incandescent lamp.
Reagents:
1. H3PO4, 85%.
2. H2SO4, concentrated (96%)
3. NaCl , solid.
4. Standard 0.167M K2Cr2O7:- Dissolve 49.04 g of dried (105oC) K2Cr2O7 in water and dilute to 1 L.
5. 0.5M Fe2+ solution: Dissolve 196.1 g of Fe(NH4)2(SO4)•6H2O in 800 mL of water containing 20 mL of concentrated H2SO4 and dilute to 1 L. The Fe2+ in this solution oxidizes slowly on exposure to air so it must be standardized against the dichromate.
6. Ferroine indicator: Slowly dissolve 3.71 g of o-phenanthroline and 1.74 g of FeSO4•7H2O in 250 mL of water.
Procedure:
1. Weigh out 0.10 to 2.00 g dried soil (ground to <60 mesh) and transfer to a 500-mL Erlenmeyer flask. The sample should contain 10 to 25 mg of organic C (17 to 43 mg organic matter). For a 1 g soil sample, this would be 1.2 to 4.3% organic matter. Use up to 2.0 g of sample for light colored soils and 0.1 g for organic soils.
2. Add 10 ml K2Cr2O7 solution by means of pipette .
3. Add 20 mL of concentrated H2SO4 by means of a dispenser and swirl gently to mix of the flask out of the solution. Avoid excessive swirling that would result in organic particles adhering to the sides of the flask out of the solution.
4. Allow to stand for 30 minutes. The flasks should be placed on an insulation pad during this time to avoid rapid heat loss.
5. Dilute the suspension with about 200 mL of water to provide a clearer suspension for viewing the endpoint.
6. Add 10 mL of 85% H3PO4, using a suitable dispenser, and 0.2 g of NaCl. The H3PO4 and NaCl is added to complex Fe3+ which would interfere with the titration endpoint.
7. Add 10 drops of ferroin indicator. The indicator should be added just prior to titration to avoid deactivation by adsorption onto clay surfaces.
8. Titrate with 0.5 M Fe2+ to a burgundy endpoint. The color of the solution at the beginning is yellow-orange to dark green, depending on the amount of unreacted Cr2O72- remaining, which shifts to a turbid gray before the endpoint and then changes sharply to a wine red at the endpoint Use of a magnetic stirrer with an incandescent light makes the endpoint easier to see in the turbid system (fluorescent lighting gives a different endpoint color). Alternatively use a Pt electrode to determine the endpoint after step 5 above. This will eliminate uncertainty in determining the endpoint by color change. If less than 5 mL of Fe2+ solution was required to back titrate the excessCr2O72- there was insufficient Cr2O72- present, and the analysis should be repeated either by using a smaller sample size or doubling the amount of K2Cr2O7 and H2SO4.
9. Run a reagent blank using the above procedure without soil. The blank is used to standardize the Fe2+ solution daily.

Calculations :-
For organic carbon= C = (B-S) x M of Fe2+ x 12 x 100g soil x 4000
Where:
B = mL of Fe2+ solution used to titrate blank
S = mL of Fe2+ solution used to titrate sample
12/4000 = Milliequivalent weight of C in sample
Date-07-07-2021
Estimation of calcium and magnesium by EDTA method
Introduction :- Calcium and Magnesium are micronutrients which are required in trace amounts to plants . Calcium is necessary for plants for synthesis of cell wall ,and magnesium is necessary cofactor for metabolism of plants . Hence it is necessary to estimate Calcium and Magnesium content in soil . We performed this experiment 5 times , some problems were observed such as standardization of chemicals like EDTA , adjusting the normality of NaOH solution . After constant repeatability we got the following procedure .
Aim :- To estimate calcium and magnesium from given soil sample by EDTA method
Reagents :-
A. Buffer Solution (NH4Cl-NH4OH) :-Dissolve 67.5 g ammonium chloride in 570 mL concentrated ammonium hydroxide, and transfer the solution to a 1-L volumetric flask, let it cool, and bring to volume with DI water.
B. Eriochrome Black Indicator:- Dissolve 0.5 g Eriochrome black with 4.5 g hydroxylamine hydrochloride in 100 mL ethyl alcohol (95%). Prepare a fresh batch every month.
C. Ethylene diamine tetra acetic Acid Solution (EDTA):- 0.01 N Dissolve 2 g ethylene diamine tetra acetic acid, and 0.05 g magnesium chloride (MgCl2) in DI water, and bring to 1-L volume with DI water.
D. Sodium Hydroxide Solution (NaOH):- 2 N Dissolve 80 g sodium hydroxide in about 800 mL DI water, transfer the solution to a 1-L volume, cool, and bring to volume with DI water.
E. Ammonium Purpurate Indicator (C8H8N6O6) :- Mix 0.5 g ammonium purpurate (Murexoid) with 100 g potassium sulfate (K2SO4).
F. Standard Stock Calcium Chloride Solution (CaCl2.2H2O):- 0.01 N Dissolve 0.5 g pure calcium carbonate (CaCO3 dried for 3 hours at 100°C), 31 UAE University in 10 mL 3 N hydrochloric acid and bring to1-L volume with DI water. This can also be prepared by dissolving 0.735 g calcium chloride dihydrate (CaCl2.2H2O) in 1-L volume with DI water.
G. Ammonium acetate solution :- Dissolve 77 grams of ammonium acetate in 80 ml of distilled water and bring volume up to 100 ml .
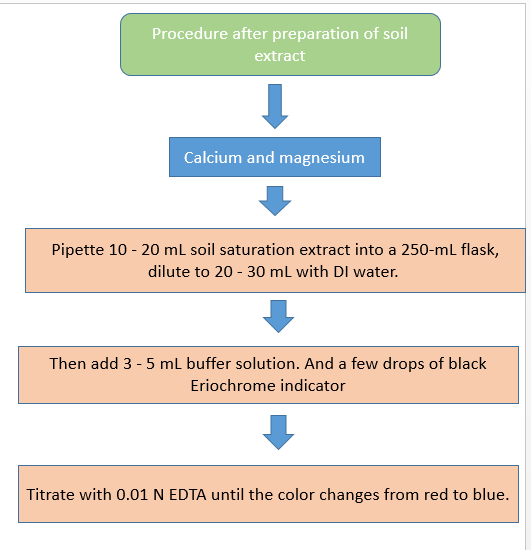
Procedure :-
A . Preparation of soil extract :-
- Weigh 50 grams of soil and add 200 ml of 40% ethanol to it .
- Filter the mixture and discard the filtrate and add ammonium acetate solution to it .
- Incubate the mixture overnight and use it as soil extract .

B. CALCIUM

C . CALCIUM AND MAGNESIUM


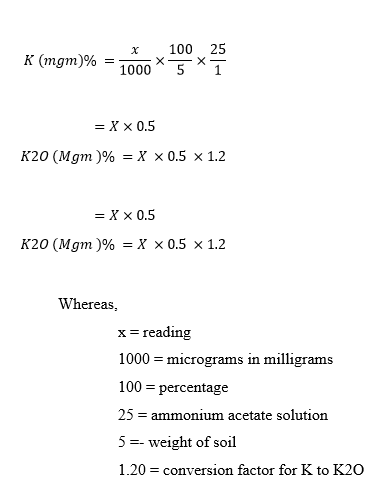
Calculations :-
Ca or Ca + Mg (meq/L) = (V – B) × N × R × 1000 / Wt of soil sample taken
for mg = ( ca+mg ) – ca
Where:-
V = Volume of EDTA titrated for the sample (mL)
B = Blank titration volume (mL)
R = Ratio between total volume of the extract and extract volume used for titration.
N = Normality of EDTA solution
Date-16-07-2021
Total Nitrogen Determination by Kjeldahl Method
Introduction:- Nitrogen is a macronutrient and is very important for plant growth . but too much nitrogen is just as dangerous . Nitrogen can also cause a lot of environmental damage in groundwater and in oceans . Hence it is necessary to estimate nitrogen content in soil.
We perform this experiment at least 11 times in our laboratory . Many problems were faced like there was a mistake for selection of trapping solution of ammonia , also we faced a problem in titration and adjusting the normality of sodium hydroxide solution , there was also problem in preparation of tashiro indicator and adjusting color of trapping solution After constant repeatability of this experiment we got excellent results and desired endpoint . The SOP for nitrogen is mentioned below .
Aim :- To estimate total nitrogen content from standard Glycine
Reagents :-
- Sulfuric acid , 95% , reagent grade
- Catalyst (copper sulphate and potassium sulphate ):- Add 5 g of potassium sulphate and 2 g copper sulphate
- Glycine powder
- Sodium hydroxide 50% :- Dissolve 50 g sodium hydroxide in 50 ml DW
- Sulphuric acid solution 0.5 M:- Dissolve 2.4 ml of 98% Sulphuric acid in 10 ml distilled water and make volume 100 ml .
- Hydrochloric acid 0.25 N :-Dissolve 2.17 ml of HCl in 100 ml DW
- Sodium carbonate solution :- For standardization of HCl solution
PROCEDURE
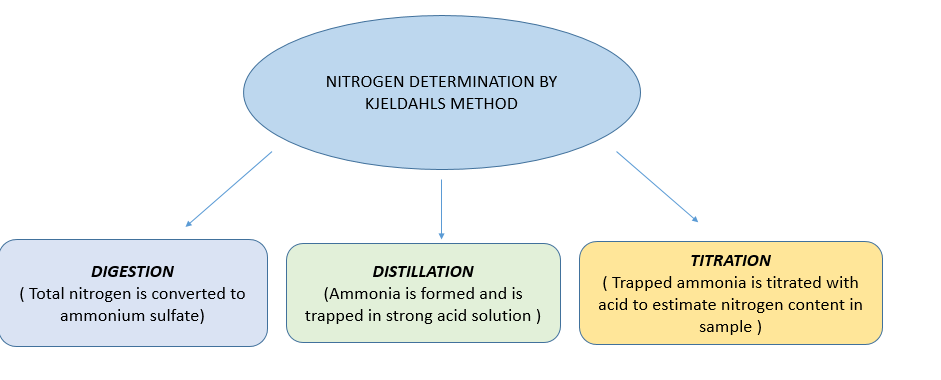
PART A :- DIGESTION
- Weigh around 1 grams of dry glycine in the digestion flask and add 2 grams of catalyst and add 20 ml sulphuric acid (95%).Add glass beads in the flask to avoid excess heating .
- Digest the content in flask for 30-40 mins until the temp raises up to 160 degree Celsius .
- Color changes from blue to colorless.
PART B :- DISTILLATION
- Add 100 ml distilled water in the digestion flask and let it cool .
- Add 50 ml 50 % sodium hydroxide solution to the flask and check pH
- Attach the flask to the distillation unit and collect the distillate in 0.5 M Sulphuric acid solution .

Note:- BACK TITRATION :-
1.Prepare 0.5 molar NaOH by adding 2gm NaOH in 98 ml D/W .
2.Then titrate it with trapping solution in which ammonia is trapped to neutralize excess acid present in trapping solution
3.If you used boric acid as a trapping solution then there is no need to back titrate it .
PART C :- TITRATION
- Add 6-7 drops of tashiro indicator to the distillate containing flask and titrate it with 0.25M HCl solution
- End point is bluish to a slight violet colour .


Problems occurred during performing this experiment :-
Trial 1 :- We used boric acid as a trapping solution , which is a weak acid and boric acid was unable to trap ammonia in it and bubbles were seen which were indicating loss of nitrogen .

Trial 2 :- We used 0.25 molar HCl solution as a titrant and as boric acid shows negative results we decided to take strong acid as a trapping solution which was 0.5 molar sulfuric acid . Now problem was color change was not observed and endpoint was not seen due to we were not performing back titration . As a result end point was not seen .

CALCULATIONS :-
% NITROGEN = (ml standard acid – ml blank) x N of acid x 1.4007 / weight of sample taken
NOTE :- FOR STANDARDISATION OF HCl :-
- Weigh approximately previously heated 1.4 gm of sodium carbonate solution and make it volume to 100 ml .
- Add 2-3 drops of methyl red indicator to it .
- Titrate it with 0.25 M HCl solution
- End point is faint pink .
DATE:- 06/08/2021
ESTIMATION OF TOTAL NITROGEN FROM AZOLLA
Sample used for Estimation :- Dried azolla powder

Procedure :- As mentioned above
Result :– The Nitrogen content in Azolla was 8.75 %.
DATE :- 16-08-2021
ESTIMATION OF TOTAL NITROGEN FROM ONION BHAJI SAMPLE :-
Need of this experiment :- We are trying to prove that there is lot of proteins content present in bhaji then glucose biscuits .
SAMPLE USED FOR ESTIMATION :- Dried powder of onion bhaji
Procedure to Dry bhaji :-
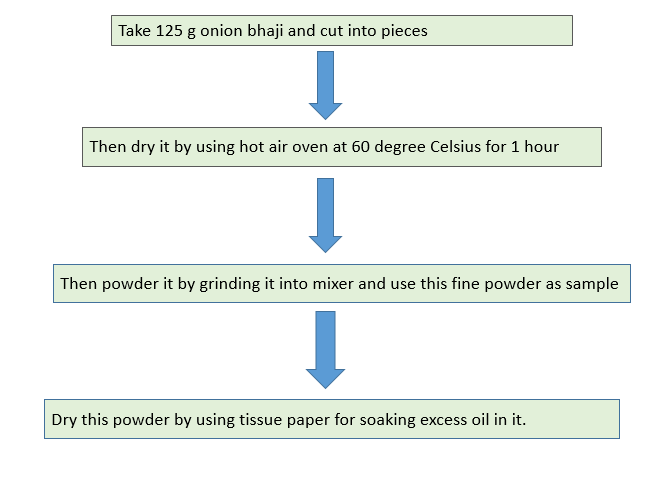



So by using this powder we can estimate total nitrogen content by using kjeldahls method.
Problem in trial 1 :- in this trial we used directly oven dried onion bhaji powder and the powder was so oily . hence in this trial we faced problem of production of excess bubbles during digestion of this powder .
Then as a solution to this problem of presence of excess oil in bhaji powder we decided to soak this powder in tissue paper to remove excess oil form it .
This trial was successful due to removal of excess oil from it .
Result :- Total nitrogen content in onion bhaji is 5.95%.
Total Nitrogen Estimation from Glucose Biscuit sample
Need of experiment:- We are trying to prove that bhaji is protein rich food then glucose biscuits
STEP 1 :- To measure LOD of given glucose biscuit sample :-
Initial weight of sample :- 5.82 gm ( with tray )
final weight of sample :- 5.76 gm ( with tray )
So , according to calculations biscuit sample contains 1.37 % moisture
| Time | Weight of sample |
| 12:30 | 5.82 |
| 1:30 | 5.80 |
| 2:30 | 5.78 |
| 3:30 | 5.78 |
| 4:30 | 5.76 |
| 5:30 | 5.76 |
Procedure :- As per mentioned above .
Result :- Nitrogen content in given sample is
DATE :- 29-08-2021
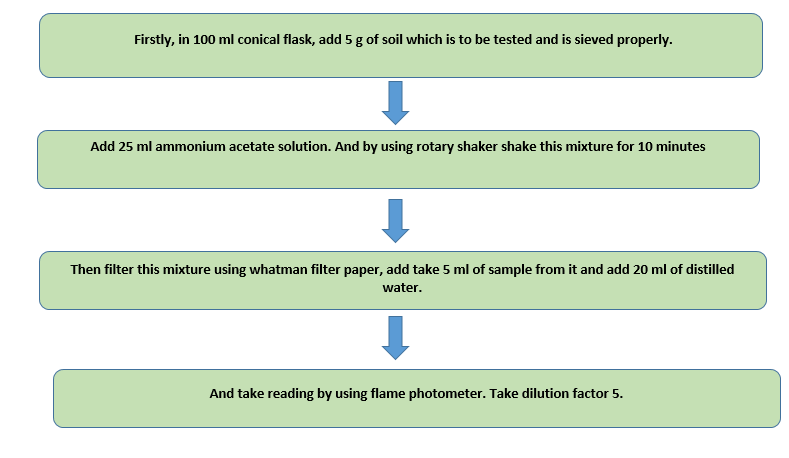
Estimation of Potassium in soil by Flame Photometer
Introduction :- Potassium is an essential plant nutrient and is required in large amounts for proper growth and reproduction of plants. . It affects the plant shape, size, color, taste and other measurements attributed to healthy produce. Plants absorb potassium in its ionic form, K+. The production of ATP can regulate the rate of photosynthesis. Potassium also helps regulate the opening and closing of the stomata, which regulates the exchange of water vapor, oxygen and carbon dioxide. . Other roles of K include: Increases root growth and improves drought resistance.
Aim :- To estimate amount of available Potassium in given soil sample .
Reagents :-
- Ammonium acetate solution:- Dissolve 77 g in 900 ml in distilled water and to adjust pH 7.0 add 3 N sodium hydroxide solution . And adjust volume up to 1 liter .
- Standard potassium chloride solution (1000ppm) :- Dissolve 1.908 g potassium chloride solution in 1 liter distilled water . We get 1000 ppm solution. , then pipette out 10 ml ,5 ml , 1 ml respectively and prepare 10 , 50 , 100ppm solution .This solutions are used for calibration of flame photometer .
- Procedure :-


Calculations :-