Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth’s hydrosphere and the fluids of most living organisms. It is vital for all known forms of life, even though it provides no calories or organic nutrients.
Water Hardness
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, largely calcium and magnesium. … When hard water is heated, such as in a home water heater, solid deposits of calcium carbonate can form.
Objective
1. To determine the concentrations of Ca2+(aq) and Mg2+(aq) ions in a sample water.
2. To compare experimental results with the concentrations of the metal ions with standerd.
Introduction
The ions involved in water hardness, i.e. Ca2+(aq) and Mg2+(aq), can be determined by titration with a chelating agent, ethylene diaminetetraacetic acid (EDTA), usually in the form of disodium salt (H2Y2-). The titration reaction is:
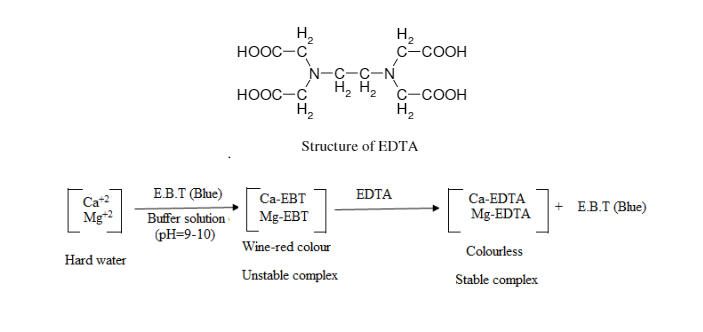
Eriochrome Black T is commonly used as indicator for the above titration. At pH 10, Ca2+(aq) ion first complexes with the indicator as CaIn+(aq) which is wine red. As the stronger ligand EDTA is added, the CaIn+(aq) complex is replaced by the CaY2-(aq) complex which is blue. The end point of titration is indicated by a sharp colour change from wine red to blue.
Titration using Eriochrome Black T as indicator determines total hardness due to Ca2+(aq) and Mg2+(aq) ions. Hardness due to Ca2+(aq) ion is determined by a separate titration at a higher pH, by adding NaOH solution to precipitate Mg(OH)2(s), using hydroxynaphthol blue as indicator
Experimental Procedures
Part A: Determination of total hardness
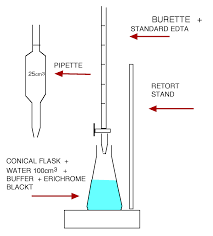
1. Pipette 50 cm3 mineral water into a conical flask. 2. Add 2 cm3 buffer solution followed by 3 drops of Eriochrome Black T indicator solution. 3. Titrate with 0.01 M EDTA until the solution turns from wine red to sky blue with no hint of red (save the solution for colour comparison). 4. Repeat the titration to obtain two concordant results.
Part B: Determination of concentration of Ca2+(aq) ions
1. Pipette 50 cm3 of mineral water into a conical flask. 2. Add 30 drops of 50% w/v NaOH solution, swirl the solution and wait for a couple of minutes to completely precipitate the magnesium ions as Mg(OH)2(s). 3. Add a pinch of hydroxynaphthol blue (exact amount to be decided by the intensity of the resulting coloured solution) and titrate with 0.01 M EDTA until it changes to sky blue (save the solution for colour comparison). 4. Repeat the titration to obtain two concordant results.


Calculation

Tap water= 50ml, Burrete reading=8 ml.
Total hardness, mg/L as CaCo3 = 8*1*1000/50 =160 mg/L




