What is Kjeldahl Method ?
The Kjeldahl method was developed in 1883 by a brewer called Johann Kjeldahl. A food is digested with a strong acid so that it releases nitrogen which can be determined by a suitable titration technique. The amount of protein present is then calculated from the nitrogen concentration of the food. The same basic approach is still used today, although a number of improvements have been made to speed up the process and to obtain more accurate measurements. It is usually considered to be the standard method of determining protein concentration. Because the Kjeldahl method does not measure the protein content directly a conversion factor (F) is needed to convert the measured nitrogen concentration to a protein concentration. A conversion factor of 6.25 (equivalent to 0.16 g nitrogen per gram of protein) is used for many applications, however, this is only an average value, and each protein has a different conversion factor depending on its amino-acid composition. The Kjeldahl method can conveniently be divided into three steps: digestion, neutralization and titration.
Introduction/ Background/Need of Project :
Proteins are polymers of amino acids. Proteins are important constituents of foods. Nitrogen is important component of protein. each protein has unique nitrogen content. Determination of Nitrogen is important to Calculate Protein content. Kjeldahl Method is used for Determination of Nitrogen We can Determine Nitrogen, Ammonia & Nitrate also, Soil Analysis, waste water analysis can be determine using Kjeldahl Method.
Why we Need to set up Kjeldahl method ?
Proteins are polymers of amino acids. Twenty different types of amino acids occur naturally in proteins. Proteins differ from each other according to the type, number and sequence of amino acids. Proteins are important constituents of foods for a number of different reasons. Ammonia was trapped by last experiments so We decided to setup procedure up to mark. this method will be use in determination of Proteins in BSF ( Black Soldier Fly ), Azolla, Soil samples, milk and soyabean etc.
Objectives :
To standardize the Procedure of determination of Nitrogen by Kejeldahl method This is necessary for total Nitrogen content will be helpful for protein analysis as well as waste water analysis including aquaponics.
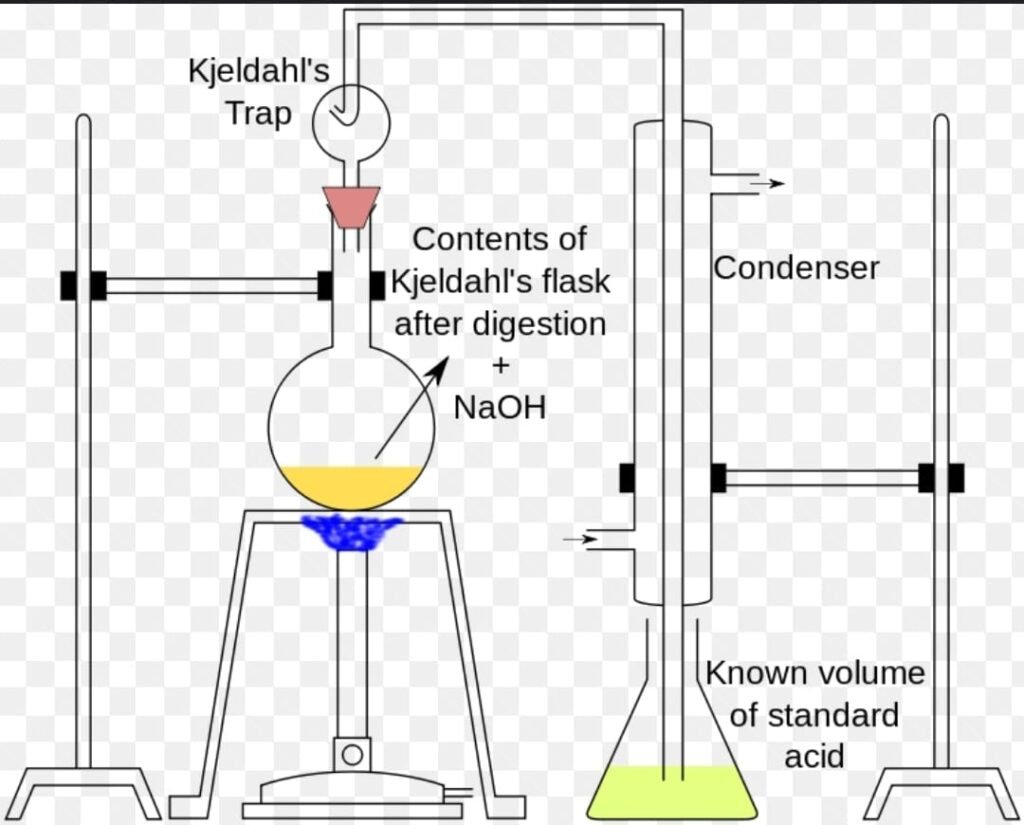
Apparatus :
- Digestion flask
- Kjedahl heating apparatus
- bends
- cones
- joints
Reagents :
- Sulphuric acid, 95%, reagent grade
- Catalyst 2 gm (copper sulfate and potassium sulfate ):- Add 5g of potassium sulfate and 2g copper sulfate
- Sodium hydroxide 50% :- Dissolve 25 g sodium hydroxide in 50 ml DW
- Sulphuric acid solution 0.5 M
- Hydrochloric acid 0.25 M
- Sodium Carbonate Solution : 0.5 M
Procedure :

1. Digestion
The digestion procedure is to break all nitrogen bonds in the sample and convert all of the organically bonded nitrogen into ammonium ions (NH4+). Organic carbon and hydrogen form carbon dioxide and water. the sample is mixed with sulfuric acid at temperatures between 80 and 100 degree Celcius. The higher the temperature used, the faster digestion can be obtained. The speed of the digestion can be greatly improved by the addition of salt and catalysts. Potassium sulfate is added in order to increase the
boiling point of sulfuric acid and catalysts are added in order to increase the speed and efficiency of the digestion procedure.
Protein (-N) + H2SO4 ————- (NH4)2SO4 + CO2 + H2O
2. Distillation
After the digestion has been completed the digestion flask is connected to a recieving flask by a tube. The solution in the digestion flask is then made alkaline by addition of sodium hydroxide, which converts the ammonium sulfate into ammonia gas
(NH4)2SO4 + 2 NaOH ————- 2NH3 + 2H2O + Na2SO4
The ammonia gas that is formed is liberated from the solution and moves out of the digestion flask and into the receiving flask – which contains an excess of boric acid. The low pH of the solution in the receiving flask converts the ammonia gas into the ammonium ion, and simultaneously converts the boric acid to the borate ion.
H2SO4(total) + 2NH3 ————-SO42- + 2 NH4+
3.Titration
When using sulfuric acid standard solution as absorbing solution, the residual sulfuric acid (the excess not reacted with NH3) is titrated with sodium hydroxide standard solution and by difference the amount of ammonia is calculated. This titration is called back titration.
H2SO4(total) + 2NH3—————– SO4 + 2NH4
Methodology :
PART A: – DIGESTION
- Weighed around 1 grams of dry glycine in the digestion flask and added 2g of catalyst and add 20 ml sulphuric acid (95%). Added glass beads in the flask to avoid excess heating.
- Digested the content in flask for 30-40 min until the temperature raises up to 160 degree Celsius.
- Color changes from blue to colorless.

PART B: – DISTILLATION
- Added 100 ml distilled water in the digestion flask and let it
cool. - Add 50 ml 50% sodium hydroxide solution to the flask.
- Attach the flask to the distillation unit and collect the
distillate in100 ml of 0.5 M Sulphuric acid solution. - keep aside 100 ml 0.5 M sulphuric acid after distillation titrate against Na2co3.

PART C: – TITRATION
- Added 6-7 drops of methyl red indicator to the distillate containing flask and titrate it with 0.5 M Sodium Carbonate solution
- End point pink to light yellow.
- titrated two solutions ammonia trapped solutions and100 ml of 0.5 M H2SO4 solution kept aside.

%𝑵 Calculation :
(𝒎𝒍 𝒔𝒕𝒂𝒏𝒅𝒂𝒓𝒅 𝒂𝒄𝒊𝒅 − 𝒎𝒍 𝒐𝒇 𝒃𝒍𝒂𝒏𝒌) × 𝑵 𝒐𝒇 𝒂𝒄𝒊𝒅 × 𝟏. 𝟒𝟎𝟎𝟕
𝑾𝒆𝒊𝒈𝒉𝒕 𝒐𝒇 𝒔𝒂𝒎𝒑𝒍𝒆 𝒊𝒏 𝒈𝒓𝒂𝒎𝒔
Where,
N = Normality of acid used
Blank = NaOH required to back titrate excess
H2SO4.




Conclusion :
- We set Procedure to determine Nitrogen with Kjeldahl method.
- Changed titrating solution primary standard for Accurancy.
- added in set up Addition glass funnel for prevent loss of ammonia ( gas).
By repeating this procedure total protein content of various samples
| Sr. No. | Sample | protein % |
| 1. | Sweet corn waste | 3.1% |
| 2. | corn silage | 7.55% |
| 3. | Sprouting puran | 55% |
| 4. | non sprouting puran | 35% |
| 5. | chana dal powder | 18% |
| 6. | dhokla | 3.112% |
Conclusion :
Protein is not easy to digest. make sprouts can break bonds of protein molecules present in sample hence it becomes very easy to digest. chana dal has around 18% proteins present , non sprouting puran has 35 % And sprouting puran has almost 55% protein content in it. hence srouting puran is best source of protein material for us.
The calculations for % nitrogen or % protein must take into account which type of receiving solution was used and
any dilution factors used during the distillation process. In the equations below, “N” represents normality. “ml blank”
refers to the millilitres of base needed to back titrate a reagent blank if standard acid is the receiving solution, or
refers to millilitres of standard acid needed to titrate a reagent blank if boric acid is the receiving solution.
If it is desired to determine % protein instead of % nitrogen, the calculated % N is multiplied by a factor, the magnitude of the factor depending on the sample matrix. Many protein factors have been developed for use with various types of samples. Here you can see the % Nitrogen, the Protein factor and the % Protein for different types of food:
| Type | Food | % Nitrogen | Factor | % protein |
| Cereals, pasta | Brown Rice | 1.3 | 6.25 | 7.9 |
| Wheat flour, whole- grain | 2.4 | 5.7 | 13.7 | |
| Macaroni, spaghetti | 1.9 | 5.7 | 11.0 | |
| Pulses, nuts and seeds | Red beans | 3.4 | 6.25 | 21.2 |
| Soy and soy products | 6.3 | 5.71 | 36.0 | |
| Almonds | 4.9 | 5.18 | 25.3 | |
| Peanuts | 4.8 | 5.46 | 26.0 | |
| Nuts | 2.9 | 5.3 | 15.2 | |
| Sunflower seeds | 3.2 | 5.3 | 17.2 | |
| Dairy products | Milk | 0.5 | 6.38 | 3.3 |
| Cheese Cheddar | 3.9 | 6.38 | 24.9 | |
| Butter | 0.3 | 6.38 | 2.0 | |
| Yogurt | 0.8 | 6.38 | 5.3 | |
| Meat, poultry, fish | Beef | 3.0 | 6.25 | 18.5 |
| Chicken, breast meat | 3.7 | 6.25 | 23.1 | |
| Egg | 2.0 | 6.25 | 12.5 | |
| Fish | 2.6 | 6.25 | 16.0 |
.
Date : 03/07/2023

Calculations :
sodium carbonate solution (0.5 M ) required for titrating 0.5 M sulphuric acid = 113.3 ml
After trapping of ammonia gas sodium carbonate solution (0.5 M ) required for titrating 0.5 M sulphuric acid = 117.6 ml
117.6-113.3 =4.3 ml
If 1000 ml contains 17 ammonia then how much in 4.3 ml ?
1000 = 17
4.3 = ?
(4.3 x 17)/1000 =0.073 gm
17 ammonia equivalent to 14 nitrogen
17 = 14
0.073 = ?
(0.073 x 14)/17 = 0.060 gm
0.060 x 5.95 = 0.357 gm =357 mg of protein in 2 gm of rice and dal
2000 mg = 357 mg
100 = ?
17.8 % protein present in rice and moong dal.





