Point discuss on brainstorming in the presence of 24/04/2021
Dr. Arun Dixit, Prasad sir, Sanket sir.
- Energy calculation for an existing system.
- Finding material with high thermal resistance for cutting the excess use of energy in form of sensible heat and losses.
- Alternative heat source economical than Electricity.
- Discussion on designs base on partial plasma combustion and pyrolisis.
- Other ways for sanitary napkin disposal (Bio-Chemical Treatment).
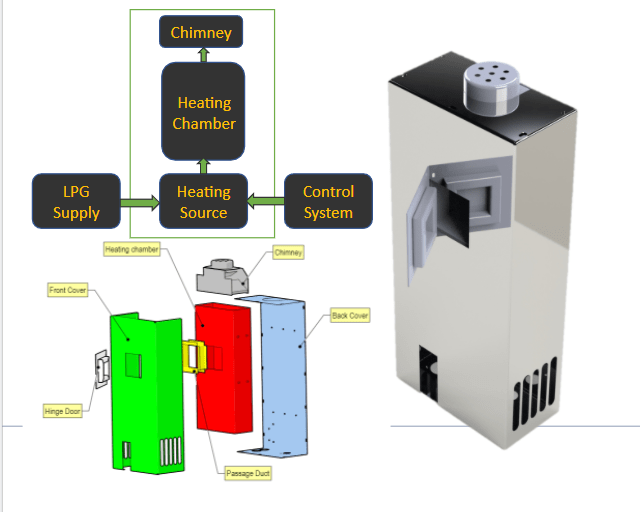
Analysis of present design of Amol Khamkar prototype v3.2
1.Energy calculation for an existing system.
The average sanitary napkin comprises 48% fluff pulp, 36% PE, PP, and PET, 7% adhesives, 6% superabsorbent, and 3% release paper.
ASSUMING
50%=PET
50%= cellulos
calorific value cotton =16 MJ/kg
calorific value PET= 22 MJ/kg
Calorific Value of cellulos=16 MJ/Kg
=16*{(103 *(KJ)} /1*{103 g}
=16KJ/g
Calorific Value of PET =22 MJ/Kg
=22*{(103 *(KJ)} /1*{103 g}
=22KJ/g
Calorific Value( KJ/kg) = Heat Produse ( KJ) / Amount of fule (Kg)
Heat Produse ( KJ) = Calorific Value( KJ/kg) * Amount of fule (Kg)
For cellulos 50% (cotton)
Heat Produse ( KJ) = 16KJ/g * (8/2) g
=64 KJ
For PET 50%
=22KJ/g * (8/2)g
=88 KJ
Total heat energy produce by complet burning of 8g pad
= Heat Produse (cellulose) + Heat Produse (PET)
=64KJ +88KJ
=152KJ
( from literature)
The energy required for pyrolysis /pad=0.216 KJ/g
Energy gain from pyrolysis /pad =22 KJ/g
Calculation of specific heat required for heating MS chamber : (Heat Source medium Ceramic IR heaters).
Parameters :
- Iner tempraur of wall=450 oc
- Outertempratur of wall=150 oc
- Specific heat of MS=0.468 KJ/Kg k
- Mass =15Kg
specific heat capacity ‘Cp‘ for MS chamber:
Q=m*Cp*dT
=15 kg *0.4680 KJ/Kg.K *(450.0-150.0)K
=2106KJ
= 2106 * (0.27W) ………………….{ 1KJ=0.27W}
=0.56862KW
=0.56862 Unit …………………………{1KW=1 Electric Unit}
=0.56862 UNITS * (10 Ruppes/Unit)
=5.6862 Rupees
2. Alternative heat source cheaper and economical than Electricity. (Heat Source medium LPG ) .
Parameters :
- Heat Produse ( KJ) =2106KJ
- The calorific value of LPG=11900 K.Cal/kg
Calorific Value( KJ/kg) = Heat Produse ( KJ) / Amount of fule (Kg)
Amount of fule (Kg) = Heat Produse ( KJ) / Calorific Value( KJ/kg)
=2106KJ / 11900 K.Cal/kg
=2106KJ / 11900 (4.18 KJ/Kg)
=0.0423 Kg
In ₹
=0.0423 Kg * (45 Rupees/Kg)
=1.9035 ₹
The energy required for vaporization of water per pad from 27 oC
Specific heat capacity of water =4.184 j/g-k
=1 cal/g oC
Specific heat required for Water
Q=m*Cp*dT
=7.132g *1cal/g-K *|(100-27)|K
=7.132g *4.184J/g-K *|(100-27)|K
29.840J/K * 73 K
=2178.34 J
=2.17834 KJ
Heat of vaporisation QL=2260 KJ/Kg
Qv=m*L.
=7.132g*2.260KJ
=16.11832KJ
Total heat required for vaporization of water /pad from room Temperature.
= Q+ Qv
=2.17834+16.11832KJ
=18.29666KJ
3.Discussion on designs base on partial plasma combustion and pyrolisis.
Grounded Designs

Conceptual Model 1: in this model, we consider 2 chambers burning designs. insulated by pack heat-insulating walls and separated by an MS sheet with a hole at center for entering fule gases at 2nd chamber and further out pot via a small pipe above with flame on it. As it seems the 1st chamber contains a pad which will be introduced with rich fuel gas and air mixture. which will be ignited using an electrical igniter. where the pad will completely burn. In the second stage, the flue gases and particulate matter produced from the burning of pads in the 1st chamber will again introduce to a poor fuel-rich air mixture to burn them further. the assumption was that the particulated matter and flume gases produce in 1st stage get fully burn and the end product will be purely CO2 &H2O or at least nonvisible gases.
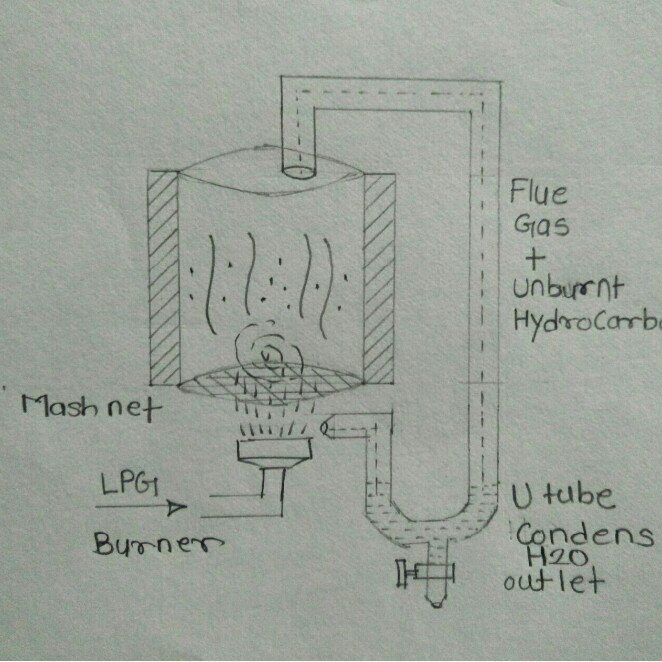
Conceptual Model 2: in this model
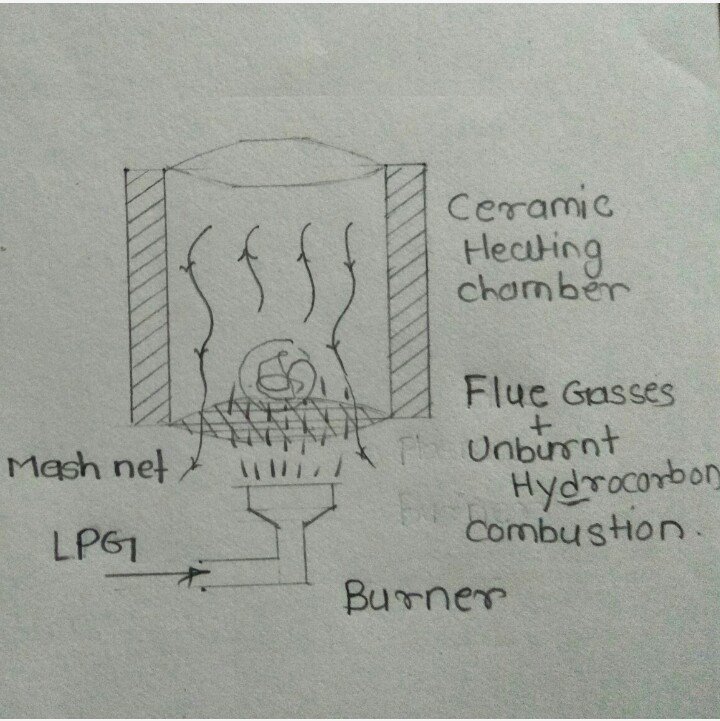
Conceptual Model 3
Experiment 1
Base on Conceptual Model 3 27/04/2021
Parameters
- The volume of pot =4.87 liters =4.87*103 cm3
- Sanitary Pad Wt
Initially dry bases =8.9g Wet bases Wt =8.9g +8.9g =17.8g
- Time
Start time =10:41 AM End time =10:45 AM
- Wt of Remaining collected =8.677g


Experiment Setup
Expectation From Experiment:
- The blue Plasma flame takes the temperature of the pad above the auto-ignition point (2010C-3040C) and the material starts combusting.
- First, the water content in the pad get vaporize and at the ignition point material started burning resulting in the formation of flue gases + Unburnt Hydrocarbon contain.
- The initial water vapor then flue gases and hydrocarbon try-except from the container bottom where they get burned by plasma.
Observation
- Complete Burning of pad did not take place due to the absence of Airflow.
- penetration of plasma flame did not observe due to small meshing size of net.
- due to small mesh size plasma flame and heat was restricted and mesh started acting like devy lamp.
- Complete vaporization of water contains.
- yellow flame at the bottom of the mesh net.

Conclusion:
mesh size of the net must be larger to prevent trapping the flame & penetration between pad and flame.
Note:
- Energy calculation during the process is not taken.
Experiment 2
Base on Experiment 1 with a large mesh Size net. 30/04/2021
Parameters
- The volume of pot =4.87 liter
=4.87*103 cm3
- Sanitary Pad Wt
Initially dry bases =8.9g
Wet bases Wt =8.9g +8.9g =17.8g
- Time
Start time = 2:27 PM
End time = 2:479PM
- Wt of Remaining collected =0.107gm
Expectation From Experiment:
- The blue Plasma flame takes the temperature of the pad above the auto-ignition point (2010C-3040C) and the material starts combusting.
- First, the water content in the pad get vaporize and at the ignition point material started burning resulting in the formation of flue gases + Unburnt Hydrocarbon contain.
- The initial water vapor, flue gases, and hydrocarbon try-except from the container bottom where they get burned by plasma.
Observation
- Complete Burning of pad did not take place due to the absence of Airflow.
- Complete vaporization of water contains.
NOTE:
Energy calculation during the process is not taken.
Experiment 3
Base on Experiment 2 with a large mesh Size net and holes at the bottom of the pot. 1/04/2021
Parameters
We replace china clay pot with Terracotta pot for making hols at the bottom (air ventilation)
The volume of pot V=πr2* h=π*11.52*21.5≈8932.72601cm2
- Sanitary Pad Wt
Initially dry pad wt =7.093g
Wet bases Wt =7.093g +7.4g(water) =14.47g
- Time
Start time =2:37 PM
End time = 2:49PM
Total Timing =12 min
- The temperature of the pot during the experiment
Outer tempratur of pot =max 75 OC
Iner tempratur of pot =max 157 OC
- Wt of Remaining char(Ash)=0.052gm
Expectation From Experiment:
- The blue Plasma flame takes the temperature of the pad above the auto-ignition point (2010C-3040C) and the material starts combusting.
- First, the water content in the pad get vaporize and at the ignition point material started burning resulting in the formation of flue gases + Unburnt Hydrocarbon contain.
- The initial water vapor .then flue gases and hydrocarbon try-except from the container bottom where they get burned by plasma.
Observation
- Complete Burning of pad takes place.
- Complete vaporization of water contains.
- a plasma of flame traps the material resulting in pyrolyzes of material.
- A green flame is observed at the top of the plasma.
- some of the materials remain to start self-ignition after turning off the gas.
- No Smoke was observed during the experiment at the bottom after turning off the flame during self-ignition of material.
- Base observe pad get self burning
NOTE:
Energy calculation during the process is not taken.
Analyses of Ash (a by-product after combustion)


Wt of Remaining char(Ash)=0.052gm
Experiment 4
Base on Experiment 3, with a change of turning on-off fuel supply within an interval. 4/05/2021

Parameters
- Terracotta pot
- MS mesh net
The volume of pot V=πr2* h=π*11.52*21.5≈8932.72601cm2
- Sanitary Pad Wt
Initially dry bases =7.132g
Wet bases Wt =7.132g +7.132g =14.227g
- Time
Start time = 11:00AM
End time =11:7AM
Time interval gap=2-3 Sec
after each 1min
- Wt of Remaining collected =NA g
Recording in presence of Dix Dr. Arun Dixit, Prasad sir.
EX 3 Observation:
- It takes 7 min (Experiment 2 takes 20 min) for the complete burning of a pad.
- In presence of a small plasma flame, there was a presence of smoke too.
- Time interval kept small (2-3sec)to avoid smoke.
- It starts self-burning and smoking as the burner turns off.
- Small pyrolytic gases seem in the first phase of interval in green shad flame at tip.
Combustion reaction for LPG & specifications
C4H10 + 13/2 O2 = 4CO2 + 5H2O
At N.T.P Volum of any one-mole gase is equal to ~24 liters
Volum of 1 mole of C4H10 =24 liters
Volum of 13/2 mole O2 =13/2*(24)liters=156 liters
Volum of 4 mole CO2 =4*(24) liters=96 liters
Volum of5 mole H2O =5*(24) liters=120 liters
The mass of 1 mole of any gas is the atomic number ‘A’ of that molecule
The mass of C4H10 ={(12)*4+10}=58 gm
The mass of 13/2 O2 ={(13/2)*(16*2)}=208 gm
The mass of 4CO2 =4{(12)*(16*2)}=176 gm
The mass of 5H2O =5{(1*2)*(16)}=90 gm
Total mass intake befor combustion is
=(A) C4H10 + (A) 13/2 O2
=58+208
=266gm
Total Volume intake befor combustion is
=(V)1 C4H10 + (V) 13/2 O2
=24+156
=180liter
Total mass after combustion is
=(A) 4CO2 + (A) 5H2O
=176gm+90gm
=266gm
Total Volume after combustion is
=(V)4CO2 + 5H2O
=96+120 liter
=216 liter
The Volum of oxygen required for combustion of one mole of C4H10,
13/2 O2=13/2*(24)liters=156 liters
Air contains 21% of oxygen
~ 100liter of Air contain 21 liters of O2
The volume of air required for combustion reaction contained 156 liter O2 is,
100liter /Air liter =21liter/156liter
The volume of Air required per mole of combustion=742.85liters
Gass mass flow rate for LPG cylinder is,
At, 500KPa 1.2Kg/hr
, 400KPa 1.0Kg/hr
, 300KPa 0.8Kg/hr
Considering gas mass flow rate of C4H10 at average =1.0Kg/hr,
Then,
C4H10 gass volum flow rate per hr at average is ,
for,
1 mole of C4H10 (58gm)=24 liters
58gm/(1kg/hr)=24liters/ V of C4H10 for 1kg /hr
V of C4H10 for 1kg/hr=(1000gm/hr * 24 liters)/58gm
=413.79 litrs /hr
Therefore, the C4H10 gas Volum flow rate at average is=413.79 liters/hr of C4H10
Now, the O2 gas mass flow rate per hour at average is,
For 58 gm of C4H10 208gm of O2 required,
For 1Kg/hr flow rate of C4H10 , O2 flow rate required given by:
58gm/(1kg/hr)=208gm/ O2 flow rate per hr
O2 flow rate per hr =3.586kg/hr
, O2 grass volume flow rate per hour at average is,
(O2 mass flow rate per mole)/( O2 mass flow rate per hr)= (O2 volume required per mole)/ (O2 volume flow rate per hr)
(208gm/mole)/(3.586Kg/hr)=(156liter/mole)/ O2 volume flow rate per hr
O2 volume flow rate per hr=(156liter/mole)* (3.586*103 gm/hr)/ (208gm/mole)
O2 volume flow rate per hr =2689.5liter/hr
Air volume flow rate per hour at average is,
O2 volume required per mole / O2 volume flow rate per hr = Air volume required per mole / Air volume flow rate per hr
156 liter/(2689.5 liter/hr)=742.85liter / Air volume flow rate per hr
Air volume flow rate per hr=742.85liter*(2689.5liter/hr)/156 liter
Air volume flow rate per hr =12,807.019liter/hr.